Biosynthesis and
biotransformation of cholesterol.
Investigation of
fatty acids and ketone bodies
metabolism.
Hormonal
adjusting and pathologies of lipids metabolism.
Synthesis of cholesterol.
There
are three stage in cholesterol synthesis. (1) acetic acid is converted to mevalonic acid, (2) mevalonic
acid is converted into squalene, and (3) squalene is converted into cholesterol.
http://www.youtube.com/watch?v=hRx_i9npTDU&feature=related
Mevalonic
acid is formed by condensation of three molecules of acetyl-CoA. The key intermediate in this process is b-hydroxy-b-methylglutaryl-CoA (HMG-CoA),
which is formed as follows:

Acetyl-CoA Acetyl-CoA Acetoacetyl-CoA

b-hydroxy-b-methylglutaryl-CoA
The enzyme is
called b-hydroxy-b-methylglutaryl-CoA synthase.
The b-hydroxy-b-methylglutaryl-CoA undergoes an irreversible two-step
reduction of one of its carboxyl groups to an alcohol group, with concomitant
loss of CoA, by the action of hydroxymethylglutaryl-CoA reductase, to
yield mevalonate:

Mevalonate
is phosphorylated by ATP, first to the
5-monophosphate ester and then to the 5-pyrophosphomevalonic acid:
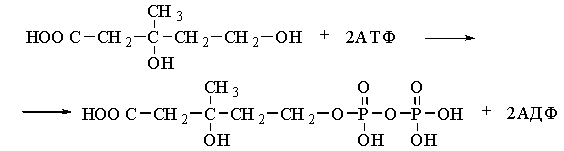
5-pyrophosphomevalonic acid
A third phosphorylation, at carbon atom 3,
yields a very unstable intermediate which loses phosphoric acid and decarboxylates to form 3-isopentenyl
pyrophosphate, which isomerizes to 3,3-dimethylallyl
pyrophosphate.

3,3-dimethylallyl
pyrophosphate
In the next several reactions 3,3-dimethylallyl pyrophosphate
is converted to squalene.
In the last stage of cholesterol biosynthesis, squalene
undergoes attack by molecular oxygen, undergoes cyclization
and lanosterol is formed. Lanosterol
is transformed to cholesterol.
Transport forms of
cholesterol in blood, content of cholesterol in blood, biological role of
cholesterol.
LDL are formed in liver and transport
cholesterol from liver to peripheral tissue. LDL is taken up by various tissues
and provides cholesterol, which the tissue utilize.
HDL picks up cholesterol from cell membranes or
from other lipoproteins. Cholesterol is converted to cholesterol esters by the lecithin:cholesterol acyltransferase
(LCAT) reaction. The cholesterol esters may be transferred to other
lipoproteins or carried by HDL to the liver, where they are hydrolyzed to free
cholesterol, which is used for synthesis of VLDL or converted to bile salts.
The content of cholesterol in blood plasma – 3-8 mmol/l.
Biological role of cholesterol:
-
building blocks of membranes;
-
synthesis of steroid hormones;
-
synthesis of bile acids;
-
synthesis of vitamin D;
-
cholesterol is often deposited on the
inner walls of blood vessels, together with other lipids, a condition known as atherosclerosis, which often
leads to occlusion of blood vessels in the heart and the brain, resulting in
heart attacks and strokes, respectively.
Transport forms of lipids
Certain lipids
associate with specific proteins to form lipoprotein
systems in which the specific
physical properties of these two classes of biomolecules are blended. In these systems the lipids
and proteins are not covalently joined but are held together largely by
hydrophobic interactions between the nonpolar portions
of the lipid and the protein components.
http://www.youtube.com/watch?v=97uiV4RiSAY
Transport lipoproteins of blood
plasma. The plasma lipoproteins are complexes in which the lipids and proteins occur
in a relatively fixed ratio. They carry water-insoluble lipids between various
organs via the blood, in a form with a relatively small and constant particle
diameter and weight. Human plasma lipoproteins occur in four major classes
that differ in density as well as particle size. They are physically
distinguished by their relative rates of flotation in high gravitational
fields in the ultracentrifuge.

http://www.youtube.com/watch?v=x-4ZQaiZry8
The
blood lipoproteins serve to transport water-insoluble triacylglycerols and
cholesterol from one tissue to another. The major carriers of triacylglyeerols
are chylomicrons and very low density lipoproteins (VLDL).
The
triacylglycerols of the chylomicrons and VLDL are digested in capillaries by
lipoprotein lipase. The fatty acids that are produced are utilized for energy
or converted to triacylglycerols and stored. The glycerol is used for
triacylglycerol synthesis or converted to DHAP and oxidized for energy, either
directly or after conversion to glucose in the liver. The remnants of the
chylomicrons are taken up by liver cells by the process of endocytosis and are
degraded by lysosomal enzymes, and the products are reused

by the cell.
VLDL is
converted to intermediate density
lipoproteins (IDL), which is
degraded by the liver or converted in blood capillaries to low density lipoproteins LDL by further digestion of
triacylglycerols.
LDL is taken up by various tissues and provides cholesterol,
which the tissue utilize
High density lipoproteins (HDL) which is synthesized by the
liver, transfers apoproteins to ehylomicrons and VLDL.
HDL
picks up cholesterol from cell membranes or from other lipoproteins. Cholesterol
is converted to cholesterol esters by the lecithin:cholesterol acyltransferase
(LCAT) reaction. The cholesterol esters may be transferred to other
lipoproteins or carried by HDL to the liver, where they are hydrolyzed to free
cholesterol, which is used for synthesis of VLDL or converted to bile salts.
Composition of the blood lipoproteins
The major components of lipoproteins are triacylglycerols, cholesterol, cholesterol esters,
phospholipids, and proteins. Purified proteins (apoproteins)
are designated A, B, C, and E.
Component Chylomicrons VLDL IDL LDL HDL
Triacylglycerol 85% 55% 26% 10% 8%
Protein 2% 9% 11% 20% 45%
Type B,C,E B,C,E B,E B A,C,E
Cholesterol 1% 7% 8% 10% 5%
Cholesterol ester 2%
10% 30% 35% 15%
Phospholipid
8% 20% 23% 20% 25%
Chylomicrons are the least dense of the blood lipoproteins because they have the
most triacylglycerol and the least protein.
VLDL is more dense than chylomicrons but
still has a high content of triacylglycerol.
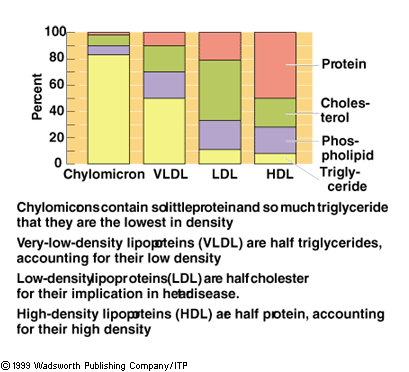
IDL, which is derived from VLDL, is more dense than chylomicrons but
still has a high content of triacylglycerol.
LDL has less triacylglycerol
and more protein and, therefore, is more dense than the IDL from which it is
derived. LDL has the highest content of cholesterol and its esters.
HDL is the most dense lipoprotein. It has the
lowest triacylglycerol and the highest protein content.
http://www.youtube.com/watch?v=XLLBlBiboJI&feature=related
Metabolism of Chylomicrons
Chylomicrons are synthesized in intestinal epithelial cells. Their triacylglycerols
are derived from dietary lipid, and their major apoprotein
is apo B-48.Chylomicrons travel through the lymph
into the blood. In peripheral
tissues, particularly adipose and muscle, the triacylglyerols
are digested by lipoprotein lipase.The
chylomicron remnants interact with receptors on liver
cells and are taken+ up by endocytosis. The contents are degraded by lysosomal enzymes, and the products (amino acids, fatty
acids, glycerol, and cholesterol) are released into the cytosol
and reutilized.
Metabolism of VLDL
VLDL is synthesized in the liver, particularly
after a high-carbohydrate meal. It is formed from triacylglycerols
that are package with cholesterol, apoproteins
(particularly apo B-100), and phospholipids and it is
released into the blood.
In peripheral
tissues, particularly adipose and muscle, VLDL triacylglycerols
are digested by lipoprotein lipase, and VLDL is converted to IDL.
IDL returns to the liver, is taken up by endocytosis,
and is degraded by lysosomal enzymes.
IDL may also be further degraded by lipoprotein
lipase, forming LDL.
LDL reacts with
receptors on various cells, is taken up by endocytosis
and is digested by lysosomal enzymes.
Cholesterol, released from cholesterol esters by a lysosomal esterase, can be used for the synthesis of cell memmbranes or bile salts in the liver or steroid hormones
in endocrine tissue.
http://www.youtube.com/watch?v=XPguYN7dcbE
Metabolism of HDL.
HDL is synthesized by the
liver and released into the blood as disk-shaped particles. The major protein
of HDL is apo A.
HDL cholesterol, obtained from
cell membranes or from other lipoproteins, is converted to cholesterol esters.
As cholesterol esters accumulate in the core of the lipoprotein, HDL particles
become spheroids.
HDL particles are taken up by
the liver by endocytosis and hydrolyzed by lysosomal enzymes. Cholesterol, released from cholesterol
esters may be packaged by the liver in VLDL and released into the blood or
converted to bile salts and secreted into the bile.
Nutrition and health


Lipids play diverse and important roles in nutrition and health. Many lipids are absolutely essential for life,

however, there is also considerable awareness that
abnormal levels of certain lipids, particularly cholesterol (in hypercholesterolemia) and, more recently, trans fatty acids, are risk factors for heart disease and other diseases. We need fats in our
bodies and in our diet. Animals in general use fat for energy storage because
fat stores 9 KCal/g of energy. Plants, which don’t move around, can afford to store food for energy in
a less compact but more easily accessible form, so they use starch (a
carbohydrate, NOT A LIPID) for energy storage. Carbohydrates and proteins store
only 4 KCal/g of energy, so fat stores over twice as
much energy/gram as other sources of energy.


We
need fats in our bodies and in our diet. Animals
in general use fat for energy storage because fat stores 9 KCal/g
of energy. Plants, which don’t move around, can afford to store food for energy
in a less compact but more easily accessible form, so they use starch (a
carbohydrate, NOT A LIPID) for energy storage. Carbohydrates and
proteins store only 4 KCal/g of energy, so fat stores
over twice as much energy/gram as fat. By the way, this is also related to the
idea behind some of the high-carbohydrate weight loss diets.
http://www.youtube.com/watch?v=-WhADd1GKtA&feature=relmfu


The human body burns carbohydrates and fats for fuel
in a given proportion to each other. The theory behind these diets is that if
they supply carbohydrates but not fats, then it is hoped that the fat needed to
balance with the sugar will be taken from the
dieter’s body stores. Fat is also is used in our bodies to a) cushion vital
organs like the kidneys and b) serve as insulation, especially just beneath the
skin.
http://www.youtube.com/watch?v=_TR8vUFP_O4&feature=related
|
Live
Blood Analysis An exciting new development in medicine, the
analysis of live blood is very different from the tests usually carried out
in laboratories, which quantify the actual levels of various components of a
sample of blood. Live Blood Analysis is a technique that allows us to view at
high magnification the quality of an individual's blood, just as it comes,
straight from the body.The technology is relatively
new to the Live Blood
Analysis is not a diagnostic procedure but a screening tool, which indicates
the effects of dietary and lifestyle factors on health. From a tiny sample,
highly magnified, the doctor can identify certain abnormalities of the blood
and lasma. Red and white blood cells and platelets
can be seen clearly and their condition assessed for signs of nutritional
deficiencies, reduced immunity, heavy metal toxicity and free radical damage.
Parasites, bacteria and yeast infections may also be visible in the sample.
These micro-organisms can be the cause of a range of chronic conditions,
which orthodox medicine is often unsuccessful at treating. in addition,
various types of fats, such as cholesterol and other crystal formations can
be identified. Further diagnostic tests may be advised. The
assessment of live blood is an important cornerstone of preventative health
care, as nutritional imbalances are apparent well before they cause
diagnosable disease states. At an early stage appropriate intervention using
nutritional medicine, together with simple dietary and lifestyle adjustments
can help mitigate against genetic tendencies to develop degenerative diseases
in later life.
Serious
health problems often positively to the holistic approach to treatment
facilitated by Live Blood Analysis.
|
A
thorough examination of the state of the live blood allows the doctor to
consider various nutritional factors that often prove to be the underlying
causes of chronic ill-health. Furthermore,
the patient's physical response to a recommended course of treatment can be
monitored visually. Improvements in the condition of the blood can be seen, sometimes
within a few days and usually within weeks. This gives patients tremendous
encouragement to continue with their regimen of supplementation and diet.
Through the practise of
Integrated Medicine, the highly effective combination of 21st
Century technology and specialist medical expertise enables an in-depth
investigation of all the elements that may be compromising an individual's
health.
Ketone bodies: structure.
http://www.youtube.com/watch?v=mLi9SEIrbuc&feature=related
In many
vertebrates the liver has the enzymatic capacity to divert some of the acetyl-CoA derived from fatty acid or pyruvate
oxidation, presumably during periods of excess formation, into free acetoacetate and b-hydroxybutyrate,
which are transported via the blood to the peripheral tissues, where they may
be oxidized via the tricarboxylic acid cyrcle.
These
compounds, together with acetone, are collectively called the ketone bodies.

acetoacetate b-hydroxybutyrate
acetone
Ketogenesis: mechanism,
localization, biological role.
Free acetoacetate, which is the primary source
of the other ketone bodies, is formed from acetoacetyl-CoA. Some of the acetoacetyl-CoA
arises from the last four carbon atoms of a long-chain fatty acid after
oxidative removal of successive acetyl-CoA residues
in the mitochondrial matrix. However, most of the acetoacetyl-CoA
formed in the liver arises from the head-to-tail condensation of two molecules
of acetyl-CoA derived from fatty acid oxidation by
the action of acetyl-CoA
acetyltransferase:

acetyl-CoA acetyl-CoA acetoacetyl-CoA
The acetoacetyl-CoA formed
in these reactions then undergoes loss of CoA, a
process called deacylation, to yield free acetoacetate
in a special pathway taking place in the mitochondrial matrix. It involves the
enzymatic formation and cleavage of b-hydroxy-b-methylglutaryl-CoA, an
intermediate which also serves as a precursor of sterols.

acetoacetyl-CoA acetyl-CoA b-hydroxy-b-methylglutaryl-CoA

b-hydroxy-b-methylglutaryl-CoA acetoacetate acetyl-CoA
The free acetoacetate so produced is enzymatically
reduced to D-b-hydroxybutyrate by the NAD-linked b-hydroxybutyrate
dehydrogenase, which is located
in the inner mitochondrial membrane.

acetoacetate b-hydroxybutyrate
The
mixture of free acetoacetate and b-hydroxybutyrate resulting from these reactions may diffuse
out of the liver cells into the bloodstream, to be transported to the
peripheral tissues.
The
mechanism of acetoacetate utilizing in tissues (ketolysis).
In
the peripheral tissues the b-hydroxybutyrate is oxidized to acetoacetate,
which is then activated by transfer of CoA from succinyl-CoA. The succinyl-CoA
required arises from the oxidation of a-ketoglutarate.

Another way of acetoacetate
activation in peripheral tissues is the direct interaction of acetoacetate with ATP and CoA-SH:

The acetoacetyl-CoA formed
in the peripheral tissues by these reactions then undergoes thiolytic
cleavage to two molecules of acetyl-CoA, which then
may enter the tricarboxylic acid cycle.
The mechanism
of the increase of ketone bodies content in blood at diabetus mellitus and starvation.
Normally the concentration of ketone bodies in the blood is rather low (10-20 mg/l), but
in fasting or in the disease diabetes mellitus, it may reach very high levels.
This condition, known as ketosis,
arises when the rate of formation of the ketone
bodies by the liver exceeds the capacity of the peripheral tissues to utilize
them, with a resulting accumulation in the blood and excretion via the kidneys
(in normal the content of ketone bodies in urine is
up to 50 mg/day).
The
utilization of acetyl-CoA in tricarboxylic
acid cycle depends on the availability of oxaloacetate
in cell. The formation of oxaloacetate depends on
quantity of pyruvate, which is formed from glucose.
In fasting or diabetus mellitus the entering of
glucose into cells is inhibited, oxaloacetate enters
the gluconeogenesis process and is not available for
the interaction with acetyl CoA in citrate synthase reaction. In this metabolic state acetyl-CoA is used for the ketone bodies
formation. The accumulation of ketone bodies is also promotted by b-oxidation
of fatty acids due to the stimulation of lipolysis in
adipose tissue in glucose starvation conditions.
The
effect of nervous system on lipid metabolism.
Sympathetic nervous system activates the
splitting of triacylglycerol (lipolysis)
and oxidation of fatty acids.
Parasympathetic nervous system promotes the
synthesis of lipids and cholesterol in organism.
Endocrine
regulation of lipid metabolism.
The effect of somatotropic
hormone on lipid metabolism:
-
stimulates lipolysis;
-
stimulates the oxidation of fatty
acids.
Prolactin.
- stimulates synthesis of lipids in mammary
glands.
Lipotropic hormone.
- stimulates the mobilization of lipids from
depot.
Thyroxine and triiodthyronine.
-
activate
the lipid oxidation and mobilization.
Insulin.
- enhances the synthesis of lipids;
- promotes the lipid storage activating the
carbohydrate decomposition;
-
inhibits the gluconeogenesis.
Glucagon.
- activates the
lipolisis;
Lipocain.
- activates the formation of phospholipids in liver
and stimulates the action of lipotropic alimentary
factors;
-
activates the oxidation of fatty
acids in liver.
Epinephrine.
-
activates the tissue lipase, mobilization of
lipids and oxidation of fatty acids.
Glucocorticoids.
-
promote the absorption of
lipids in intestine;
-
activate lipolysis;
-
activate the conversion of
fatty acids in carbohydrates.
Sex
hormones.
-
enhance the oxidation of
lipids;
-
inhibit the synthesis of
cholesterol.
Interrelationship of carbohydrate and lipid metabolism.
Interrelationship of carbohydrate metabolism with
lipid metabolism has two directions: 1-st – convertion of carbohydrates to lipids, 2-nd - convertion of lipids to
carbohydrates.
Transformation of carbohydrates to lipids.
1. Glycerol phosphate can be
produced from dihydroxiacetone monophosphate.
The last is the common intermediate product of lipid and carbohydrate
metabolism. Glycerol phosphate is the
active form of glycerol and can react with active forms of fatty acids
resulting in synthesis of triacylglycerols and
complex lipids.
2. Biosynthesis of fatty acids takes place from acetyl-CoA which is formed in oxidative decarboxilation
of pyruvate. Pyruvate is the
central intermediate product of carbohydrate metabolism.
3. Carbohydrates are also source of hydrogen atoms, which are necessary
for fatty acids synthesis. For this purpose the hydrogen atoms of reduced
coenzymes NADPH2 are used. NADPH2 are usually produced in
pentose phosphate cycle.
Transformation of lipids to
carbohydrates.
The formation of carbohydrates from other
compounds is called gluconeogenesis.
1. In b-oxidation of
fatty acids the acetyl-CoA is formed. Acetyl-CoA can’t be converted directly to pyruvate.
But it enter the tricarboxilic acid cycle and some
intermediates of this cycle can be used for gluconeogenesis.
2. Small amount of carbohydrates can be also synthesised from glycerol by means of its oxidation to dihydroxiacetone monophosphate
and glycerolaldehyde phosphate, which are the
intermediates metabolites of glycolysis.


