Metabolism
of lipids: digestion, absorption, resynthesis in the intestinal wall. Transport forms of blood lipids. Metabolism of lipids: oxidation and biosynthesis of fatty acids, triacylglycerols and phospholipids.
Lipids are
water-insoluble organic biomolecules that can be extracted from cells and
tissues by nonpolar solvents, e.g., chloroform, ether, or benzene.
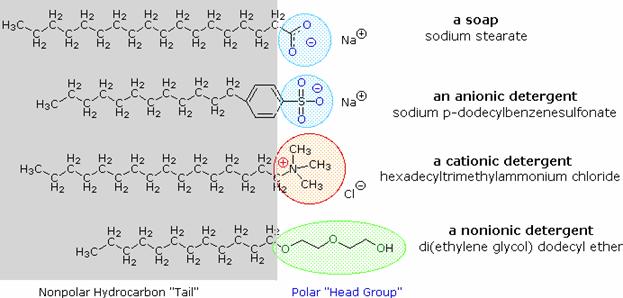
Lipids are an amphiphilic class of hydrocarbon-containing organic compounds. Lipids are
categorized by the fact that they have complicated solvation properties, giving
rise to lipid polymorphism. Lipid molecules have
these properties because they consist largely of long hydrocarbon tails which are lipophilic in nature as well as
polar headgroups (e.g. phosphate-based functionality, and/or inositol based functionality).
In living organisms, lipids are used for energy storage, serve as the
structural components of cell membranes, and constitute
important signalling molecules. Although the term
lipid is often used as a synonym for fat, the latter is in fact
a subgroup of lipids called triglycerides.
There are several different families or classes of lipids but all derive
their distinctive properties from the hydrocarbon nature of a major portion of
their structure.
Biological functions of lipids
Biological
molecules that are insoluble in aqueous solutions and soluble in organic
solvents are classified as lipids. The lipids of physiological importance for
humans have four major functions:
Lipids have several important
biological functions, serving
(1)
as
structural components of membranes,
(2)
as storage
and transport forms of metabolic fuel,
(3)
as a protective
coating on the surface of many organisms, and
(4)
(5)
as cell-surface components
concerned in cell recognition, species specificity, and tissue immunity. Some
substances classified among the lipids have intense biological activity; they include
some of the vitamins and hormones.
Although lipids are a distinct class
of biomolecules, we shall see that they often occur combined, either covalently
or through weak bonds, with members of other classes of biomolecules to yield
hybrid molecules such as glycolipids,
which contain both carbohydrate and lipid groups, "and lipoproteins, which contain both lipids
and proteins. In such biomolecules the distinctive chemical and physical properties
of their components are blended to fill specialized biological functions.
Classification of lipids
Lipids have been classified in
several different ways. The most satisfactory classification is based on their
backbone structures:
1.
Simple
lipids:
1) acylglycerols;
2) steroids;
3) waxes.
2. Complex lipids:
1)
phospholipids
a)
glycerophospholipids;
b) sphingophospholipids.
2) glycolipids
a) glycosylglycerols;
b) glycosphingolipids.
Lipids
usually contain fatty acids as components. Such lipids are called saponifiable lipids since they yield soaps
(salts of fatty acids) on alkaline hydrolysis. The other
great group of lipids which do not contain fatty acids and hence are
nonsapomfiable.
Let us first consider the structure
and properties of fatty acids, characteristic components of all the complex
lipids.
Fatty Acids
Fatty acids and glycerides
Fatty acids fill two major roles in the
body:
·
1. as the components of more complex membrane lipids.
·
2. as the major components of stored fat in the form of
triacylglycerols.
Fatty acids are long-chain hydrocarbon molecules
containing a carboxylic acid moiety at one end. The numbering of carbons in
fatty acids begins with the carbon of the carboxylate group. At physiological
pH, the carboxyl group is readily ionized, rendering a negative charge onto
fatty acids in bodily fluids.
Fatty acids that
contain no carbon-carbon double bonds are termed saturated fatty acids; those
that contain double bonds are unsaturated fatty acids. The numeric designations
used for fatty acids come from the number of carbon atoms, followed by the
number of sites of unsaturation (eg, palmitic acid is a 16-carbon fatty acid
with no unsaturation and is designated by 16:0). The site of unsaturation in a
fatty acid is indicated by the symbol and the number of the first carbon of the
double bond (e.g. palmitoleic acid is a 16-carbon fatty acid with one site of
unsaturation between carbons 9 and 10, and is designated by 16:19).
Saturated fatty acids of less than eight
carbon atoms are liquid at physiological temperature, whereas those containing
more than ten are solid. The presence of double bonds in fatty acids
significantly lowers the melting point relative to a saturated fatty acid.
The majority of body
fatty acids are acquired in the diet. However, the lipid biosynthetic capacity
of the body (fatty acid synthase and other fatty acid modifying enzymes) can
supply the body with all the various fatty acid structures needed. Two key
exceptions to this are the highly unsaturated fatty acids know as linoleic acid
and linolenic acid, containing unsaturation sites beyond carbons 9 and 10.
These two fatty acids cannot be synthesized from precursors in the body, and
are thus considered the essential fatty
acids; essential in the sense that they must be provided in the diet. Since
plants are capable of synthesizing linoleic and linolenic acid humans can
aquire these fats by consuming a variety of plants or else by eating the meat
of animals that have consumed these plant fats.
Chemically, fatty acids
can be described as long-chain monocarboxylic acids and have a general
structure of CH3(CH2)nCOOH.
The length of the chain usually ranges from 12 to 24, always with an even
number of carbons. When the carbon chain contains no double bonds, it is a saturated chain. If it contains
one or more such bonds, it is unsaturated. The presence of double bonds
generally reduces the melting point of fatty acids. Furthermore, unsaturated
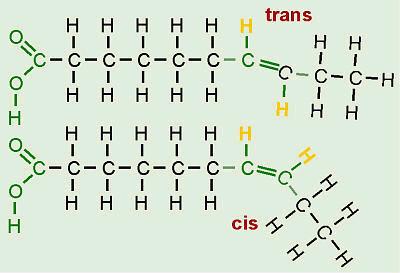
fatty acids can occur either in cis or trans geometric isomers. In naturally occurring fatty acids, the
double bonds are in the cis-configuration.
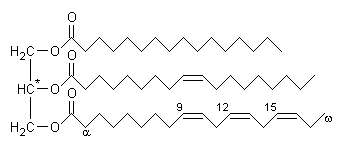
Glycerides are lipids possessing a glycerol (propan-1, 2, 3-triol) core structure
with one or more fatty acyl groups, which are fatty acid-derived chains
attached to the glycerol backbone by ester linkages. Glycerides with three acyl
groups (triglycerides or neutral fats) are the main storage
form of fat in animals and plants.
An important type of
glyceride-based molecule found in biological membranes, such as the cell's plasma membrane and the intracellular
membranes of organelles, are the
phosphoglycerides or glycerophospholipids. These are phospholipids that contain a
glycerol core linked to two fatty acid-derived "tails" by ester or,
more rarely, ether linkages and to one
"head" group by a phosphate ester linkage. The
head groups of the phospholipids found in biological membranes are
phosphatidylcholine (also known as PC, and lecithin),
phosphatidylethanolamine (PE), phosphatidylserine and phosphatidylinositol (PI). These phospholipids are subject to a
variety of functions in the cell: for instance, the lipophilic and polar ends
can be released from specific phospholipids through enzyme-catalysed hydrolysis
to generate secondary messengers involved in signal transduction. In the case of phosphatidylinositol, the head group can be enzymatically
modified by the addition of one, two or three phosphate groups, this
constituting another mechanism of cell signalling. While phospholipids are the major
component of biological membranes, other non-glyceride lipid components like sphingolipids and sterols (such as cholesterol in animal cell membranes) are also found
in biological membranes.
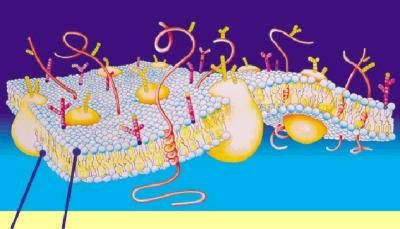
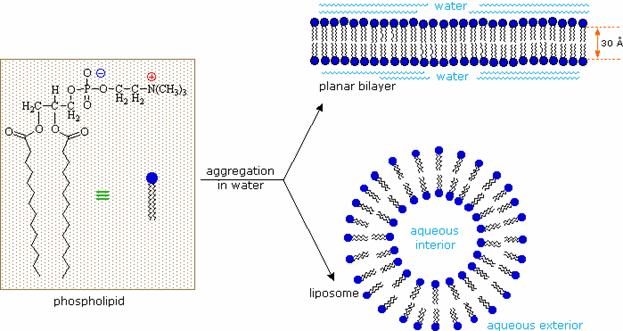
A biological membrane is a form of lipid
bilayer, as is a liposome. Formation of lipid bilayers is an
energetically-favoured process when the glycerophospholipids described above
are in an aqueous environment. In an aqueous system, the polar heads of lipids
orientate towards the polar, aqueous environment, while the hydrophobic tails
minimise their contact with water. The
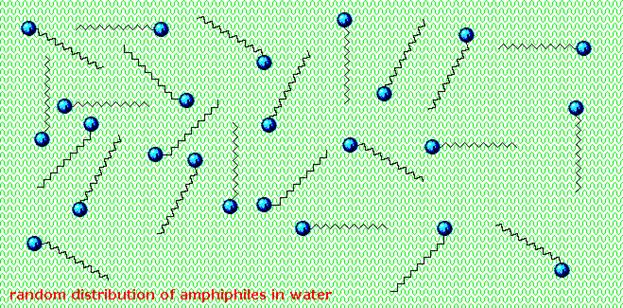
lipophilic tails of lipids (U) tend to cluster together, forming a lipid bilayer (1) or a micelle (2). Other
aggregations are also observed and form part of the polymorphism of amphiphile (lipid) behaviour. The
polar heads (P) face the aqueous environment, curving away from the water. Phase behaviour is a complicated area
within biophysics and is the subject of current academic research.
Micelles and bilayers form in the polar
medium by a process known as the lipophilic effect. When dissolving a lipophilic or amphiphilic substance in a polar
environment, the polar molecules (i.e. water in an aqueous solution) become
more ordered around the dissolved lipophilic substance, since the polar
molecules cannot form hydrogen bonds to the lipophilic
areas of the amiphphile. So, in an aqueous
environment the water molecules form an ordered "clathrate" cage around the
dissolved lipophilic molecule.
The self-organisation depends on the concentration of
the lipid present in solution. Below the critical micelle
concentration,
the lipids form a single layer on the liquid surface and are (sparingly)
dispersed in the solution. At the first critical micelle concentration (CMC-I),
the lipids organise in spherical micelles, at given points above this
concentration, other phases are observed (see lipid polymorphism).
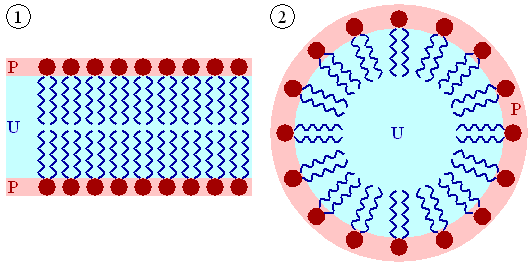
Self-organization of phospholipids. A lipid bilayer is shown on the left and a micelle on the right.
http://www.youtube.com/watch?v=CLaAPl-_rRM&NR=1
Although fatty acids occur in very large amounts as
building-block components of the saponifiable lipids, only traces occur in free
(unesterified) form in cells and tissues. Well over 100 different kinds of
fatty acids have been isolated from various lipids of animals, plants, and
microorganisms. All possess a long hydrocarbon chain and a terminal carboxyl
group. The hydrocarbon chain may be saturated, as in palmitic acid, or it may have one or more double bonds, as in oleic acid; a few fatty acids contain
triple bonds. Fatty acids differ from each other
primarily in chain length and in the number and position of their unsaturated
bonds. They are often symbolized by a shorthand notation that designates the
length of the carbon chain and the number, position, and configuration of the
double bonds. Thus palmitic acid (16 carbons, saturated) is symbolized 16:0
and oleic acid [18 carbons and one double bond (cis) at carbons 9 and 10] is
symbolized 18:1. It is understood that the double bonds are cis (see below)
unless indicated otherwise.
Some
generalizations can be made on the different fatty acids of higher plants and
animals. The most abundant have an even number of carbon atoms with chains
between 14 and 22 carbon atoms long, but those with 16 or 18 carbons predominate.
The most common among the saturated fatty acids are palmitic acid (Cis) and
stearic acid (Cis) and among the unsaturated fatty acids oleic acid (Cis).
Unsaturated fatty acids predominate over the saturated ones, particularly in
higher
plants and in animals living at low temperatures.
Unsaturated fatty acids have lower melting points than saturated fatty acids of
the same chain length. In most monounsaturated (monoenoic) fatty acids of
higher organisms there is a double bond between carbon atoms 9 and 10. In most
polyunsaturated (polyenoic) fatty acids one double bond is between carbon atoms
9 and 10; the additional double bonds usually occur between the 9,10 double
bond and the methyl-terminal end of the chain. In most types of polyunsaturated
fatty acids the double bonds are separated by one methylene group, for example,
—CH=CH—CH2—CH=CH—; only in a few types of plant fatty acids are the
double bonds in conjugation, that is, —CH=CH—CH=CH—. The double bonds of
nearly all kinds of naturally occurring unsaturated fatty acids are in the cis geometrical configuration; only a
very few are trans.
There are two kinds of fats,
saturated and unsaturated. Unsaturated fats have at least one double
bond in one of the fatty acids. A double bond happens when two electrons are
shared or exchanged in a bond. They are much stronger than single bonds. Saturated
fats have no double bonds.
Fats have a lot of energy stored up in their molecular bonds. That's why the
human body stores fat as an energy source. When it needs extra fuel, your body
breaks down the fat and uses the energy. Where one molecule of sugar only gives
a small amount of energy, a fat molecule gives off many times more.
Some naturally occuring fatty acids
|
Symbol |
Structure
|
Systemic name |
Common name |
Saturated fatty acid
|
|||
|
Ñ12:0 |
ÑÍ3(ÑÍ2)10ÑÎÎÍ |
n-Dodecanoic |
Lauric |
|
Ñ14:0 |
ÑÍ3(ÑÍ2)12ÑÎÎÍ |
n-Tetradecanoic |
Myristic |
|
Ñ16:0 |
ÑÍ3(ÑÍ2)14ÑÎÎÍ |
n-Hexadecanoic |
Palmitic |
|
Ñ18:0 |
ÑÍ3(ÑÍ2)16ÑÎÎÍ |
n-Octadecanoic |
Stearic |
|
Ñ20:0 |
ÑÍ3(ÑÍ2)18ÑÎÎÍ |
n-Eicosanoic |
Arachidic |
|
Ñ22:0 |
ÑÍ3(ÑÍ2)20ÑÎÎÍ |
n-Docosanoic |
Begenic |
|
Ñ24:0 |
ÑÍ3(ÑÍ2)22ÑÎÎÍ |
n-Tetracosanoic |
Lignoceric |
Unsaturated monoenic fatty acid
|
|||
|
Ñ16:1 |
ÑÍ3(ÑÍ2)5ÑÍ=ÑÍ(ÑÍ2)7ÑÎÎÍ |
|
Palmitooleic |
|
Ñ18:1 |
ÑÍ3(ÑÍ2)7ÑÍ=ÑÍ(ÑÍ2)7ÑÎÎÍ |
|
Oleic |
Unsaturated polienic fatty acid
|
|||
|
Ñ18:2 |
ÑÍ3(ÑÍ2)4(ÑÍ=ÑÍÑÍ2)2(ÑÍ2)6ÑÎÎÍ |
|
Linoleic |
|
Ñ18:3 |
ÑÍ3ÑÍ2(ÑÍ=ÑÍÑÍ2)3(ÑÍ2)6ÑÎÎÍ |
|
Linolenic |
|
Ñ20:4 |
ÑÍ3(ÑÍ2)4(ÑÍ=ÑÍÑÍ2)4(ÑÍ2)2ÑÎÎÍ |
|
Arachidonic |
All Lipids are hydrophobic:
that’s the one property they have in common. This group of molecules includes fats
and oils, waxes, phospholipids, steroids (like cholesterol), and some other
related compounds.
Structure of Fatty Acids
|
|
|
Fats and oils
are made from two kinds of molecules: glycerol
(a type of alcohol with a hydroxyl group on each of its three carbons) and
three fatty acids joined by
dehydration synthesis. Since there are three fatty acids attached, these are
known as triglycerides. “Bread” and
pastries from a “bread factory” often contain mono- and diglycerides as
“dough conditioners.” Can you figure out what these molecules would look like? The main
distinction between fats and oils is whether they’re solid or liquid at room
temperature, and this, as we’ll soon see, is based on differences in the
structures of the fatty acids they contain. |
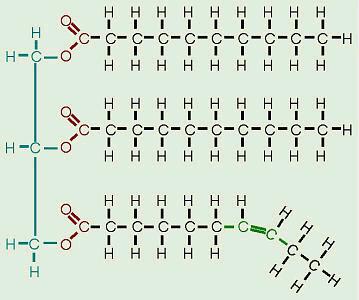
Essential
fatty acids
When weanling or immature rats
are placed on a fat-free diet, they grow poorly, develop a scaly skin, lose hair,
and ultimately die with many pathological signs. When linoleic acid is present in the diet, these conditions do not
develop. Linolenic acid and arachidonic acid also prevent these symptoms. Saturated
and monounsaturated fatty acids are inactive. It has been concluded that
mammals can synthesize saturated and monounsaturated fatty acids from other
precursors but are unable to make linoleic and linolenic acids. Fatty acids
required in the diet of mammals are called essential
fatty acids. The most abundant essential fatty acid in mammals is linoleic acid, which makes up from 10 to
20 percent of the total fatty acids of their triacylglycerols and
phosphoglycerides. Linoleic and linolenic acids cannot be synthesized by
mammals but must be obtained from plant sources, in which they are very
abundant. Linoleic acid is a necessary precursor in mammals for the
biosynthesis of arachidonic acid,
which is not found in plants.
The terms saturated, mono-unsaturated,
and poly-unsaturated refer to the number
of hydrogens attached to the hydrocarbon tails of the fatty acids as compared
to the number of double bonds between carbon atoms in the tail. Fats, which are
mostly from animal sources, have all single bonds between the carbons in their
fatty acid tails, thus all the carbons are also bonded to the maximum number of
hydrogens possible. Since the fatty acids in these triglycerides contain the
maximum possible amouunt of hydrogens, these would be called saturated fats. The hydrocarbon chains in these fatty
acids are, thus, fairly straight and can pack closely together, making these
fats solid at room temperature. Oils, mostly from plant sources, have some
double bonds between some of the carbons in the hydrocarbon tail, causing bends
or “kinks” in the shape of the molecules. Because some of the carbons share
double bonds, they’re not bonded to as many hydrogens
as they could if they weren’t double bonded to each other. Therefore these oils
are called unsaturated fats. Because
of the kinks in the hydrocarbon tails, unsaturated fats can’t pack as closely
together, making them liquid at room temperature. Many people have heard that
the unsaturated fats are “healthier” than the saturated ones. Hydrogenated vegetable oil (as in
shortening and commercial peanut butters where a solid consistency is sought)
started out as “good” unsaturated oil. However, this commercial product has had
all the double bonds artificially broken and hydrogens artificially added (in a
chemistry lab-type setting) to turn it into saturated fat that bears no
resemblance to the original oil from which it came (so it will be solid at room
temperature).
Although the specific functions of
essential fatty acids in mammals were a mystery for many years, one function
has been discovered. Essential fatty acids are necessary precursors in the
biosynthesis of a group of fatty acid derivatives called prostaglandins, hormonelike compounds which in trace amounts have
profound effects on a number of important physiological activities.
Physical and chemical properties of fatty acids
Saturated and unsaturated
fatty acids have quite different conformations. In saturated fatty acids, the
hydrocarbon tails are flexible and can exist in a very large number of conformations
because each single bond in the backbone has complete freedom of rotation.
Unsaturated fatty acids, on the other hand, show one or more rigid kinks contributed
by the nonrotating double bond(s).
Unsaturated fatty acids
undergo addition reactions at their double bonds. Quantitative titration with
halogens, e.g., iodine or bromine, can yield information on the relative
number of double bonds in a given sample of fatty acids or lipid.
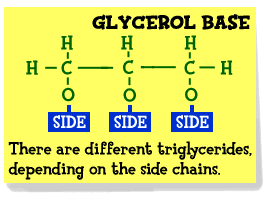
Triacylglycerols (Triglycerides)
Fat is also known as a
triglyceride. It is made up of a molecule known as glycerol
that is connected to one, two, or three fatty acids. Glycerol is the basis of
all fats and is made up of a three-carbon chain. It connects the fatty
acids together. A fatty acid is a long chain of carbon atoms connected
to each other.
Fatty acid esters of the
alcohol glycerol are called acylglycerols
or glycerides; they are
sometimes referred to as "neutral
fats," a term that has become archaic. When all three hydroxyl groups
of glycerol are esterified with fatty acids, the structure is called a triacylglycerol:

Although the name
"triglyceride" has been traditionally used to designate these
compounds, an international nomenclature commission has recommended that this
chemically inaccurate term no longer be used. Triacylglycerols are the most
abundant family of lipids and the major components of depot or storage lipids
in plant and animal cells. Triacylglycerols that are solid at room temperature
are often referred to as "fats" and those which are liquid as
"oils." Diacylgiycerols
(also called diglycerides) and monoacylgiycerols (or monoglycerides) are
also found in nature, but in much smaller amounts.
Triacylglycerols occur in many
different types, according to the identity and position of the three fatty acid
components esterified to glycerol. Those with a single kind of fatty acid in
all three positions, called simple triacylglycerols, are named after the fatty
acids they contain. Examples are tristearoylglycerol,
tripalmitoylglycerol, and trioleoylglycerol; the trivial
and more commonly used names are tristearin,
tripalmitin, and trioiein, respectively. Mixed triacylglycerols
contain two or more different fatty acids. The naming of mixed triacylglycerols
can be illustrated by the example of 1-palmitoyldi-stearoylglycerol
(trivial name, 1-palmitodistearin).
Most natural fats are extremely complex mixtures of simple and mixed
triacylglycerols.
Properties of
triacylglycerols
The melting
point of triacylglycerols is determined by their fatty acid components. In
general, the melting point increases with the number and length of the
saturated fatty acid components. For example, tripalmitin and tristearin are
solids at body temperature, whereas triolein and trilinolein are liquids. All
triacylglycerols are insoluble in water and do not tend by themselves to form
highly dispersed micelles. However,
diacylglycerols and monoacylglycerols have appreciable polarity because of
their free hydroxyl groups and thus can form micelles. Diacyl- and
monoacylglycerols find wide use in the food industry in the production of more
homogeneous and more easily processed foods; they are completely digestible and
utilized biologically. Acylglycerols are soluble in ether, chloroform,
benzene, and hot ethanol. Their specific gravity is lower than that of water. Acylglycerols
undergo hydrolysis when boiled with acids or bases or by the action of lipases,
e.g., those present in pancreatic juice. Hydrolysis with alkali, called
saponification, yields a mixture of soaps and glycerol.
Steroids
Steroids
occur in animals in something called hormones. The basis of a
steroid molecule is a four-ring structure, one with five carbons and three with
six carbons in the rings. You may have heard of steroids in the news. Many body
builders and athletes use anabolic steroids to build muscle mass. The steroids
make their body want to add more muscle than they normally would be able to. The body builders wind up stronger and
bulkier (but not faster).
Never take drugs to enhance your body. Those body builders are actually hurting
their bodies. They can't see it because it is slowly destroying their internal
organs and not the muscles. When they get older, they can have kidney and liver
problems. Some even die.
The important class of lipids called steroids
are actually metabolic derivatives of terpenes, but they are customarily
treated as a separate group. Steroids may be recognized by their tetracyclic
skeleton, consisting of three fused six-membered and one five-membered ring, as
shown in the diagram to the right. The four rings are designated A, B, C &
D as noted, and the peculiar numbering of the ring carbon atoms (shown in red)
is the result of an earlier misassignment of the structure. The substituents designated by R are often alkyl
groups, but may also have functionality. The R group at the A:B
ring fusion is most commonly methyl or hydrogen, that at the C:D fusion is
usually methyl. The substituent at C-17 varies considerably, and is usually
larger than methyl if it is not a functional group. The most common locations
of functional groups are C-3, C-4, C-7, C-11, C-12
& C-17. Ring A is sometimes aromatic. Since a number of tetracyclic
triterpenes also have this tetracyclic structure, it cannot be considered a
unique identifier.
Steroids are widely distributed in
animals, where they are associated with a number of physiological processes.
Examples of some important steroids are shown in the following diagram.
Different kinds of steroids will be displayed by clicking the "Toggle
Structures" button under the diagram. Norethindrone is a synthetic
steroid, all the other examples occur naturally. A common strategy in pharmaceutical chemistry is to take a natural
compound, having certain desired biological properties together with undesired
side effects, and to modify its structure to enhance the desired
characteristics and diminish the undesired. This is sometimes accomplished by
trial and error.
The generic steroid structure drawn above has seven chiral stereocenters
(carbons 5, 8, 9, 10, 13, 14 & 17), which means that it may have as many as
128 stereoisomers. With the exception of C-5, natural steroids generally have a
single common configuration. This is shown in the last of the toggled displays,
along with the preferred conformations of the rings.
Chemical studies of the steroids were very important
to our present understanding of the configurations and conformations of
six-membered rings. Substituent groups at different sites on the tetracyclic
skeleton will have axial or equatorial orientations that are fixed because of
the rigid structure of the trans-fused rings. This fixed orientation influences
chemical reactivity, largely due to the greater steric hindrance of axial
groups versus their equatorial isomers. Thus an equatorial hydroxyl group is
esterified more rapidly than its axial isomer.
Steroids are complex ethers of cyclic spirits sterols and fatty acids. Sterols are
derivatives of the saturated tetracylic hydrocarbon
cyclopentanoperhydrophenanthrene:
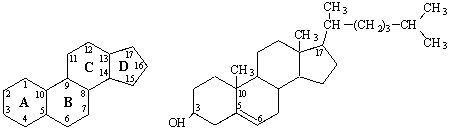
Cyclopentanoperhydrophenanthrene Cholesterol
The general structure of cholesterol
consists of two six-membered rings side-by-side and sharing one side in common,
a third six-membered ring off the top corner of the right ring, and a
five-membered ring attached to the right side of that.
The central core of this molecule, consisting of four fused rings, is
shared by all steroids, including
estrogen (estradiol), progesterone, corticosteroids such as cortisol
(cortisone), aldosterone, testosterone, and Vitamin D. In the various types of
steroids, various other groups/molecules are attached around the edges. Know
how to draw the four rings that make up the central structure.
Cholesterol is not a “bad guy!” Our bodies make about
2 g of cholesterol per day, and that makes up about 85% of blood cholesterol,
while only about 15% comes from dietary sources. Cholesterol is the precursor
to our sex hormones and Vitamin D.
Vitamin D is formed by the action of UV light in sunlight on cholesterol
molecules that have “risen” to near the surface of the skin. At least one
source I read suggested that people not shower immediately after being in the
sun, but wait at least ½ hour for the new Vitamin D to be absorbed
deeper into the skin. Our cell membranes contain a lot of cholesterol (in
between the phospholipids) to help keep them “fluid” even when our cells are
exposed to cooler temperatures.
Many people have hear the claims that egg yolk
contains too much cholesterol, thus should not be eaten. An interesting study
was done at 
A great
many different steroids, each with a distinctive function or activity, have
been isolated from natural sources. Steroids differ in the number and position
of double bonds, in the type, location, and number of substituent functional
groups, in the configuration of the bonds between the substituent groups and
the nucleus, and in the configuration of the rings in relation to each other. Cholesterol
is the most abundant steroid in animal tissues. Cholesterol and lanosterol
are members of a large subgroup of steroids called the sterols. They are steroid alcohols containing a hydroxyl group
at carbon 3 of ring A and a branched aliphatic chain of eight or more carbon
atoms at carbon 17. They occur either as free alcohols or as long-chain fatty
acid esters of the hydroxyl group at carbon 3; all are solids at room
temperature. Cholesterol melts at 150 °C and is insoluble in water but readily
extracted from tissues with chloroform, ether, benzene, or hot alcohol.
Cholesterol occurs in the plasma membranes of many animal cells and in the
lipoproteins of blood plasma. Lanosterol
was first found in the waxy coating of wool in esterified form before it was
established as an important intermediate in the biosynthesis of cholesterol in
animal tissues.
Cholesterol is the precursor
of many other steroids in animal tissues, including the bile acids, detergentlike compounds that aid in
emulsification and absorption of lipids in the intestine; the androgens, or male sex hormones; the estrogens, or female sex
hormones; the progestational hormone progesterone; and the adrenocortical hormones. Among the most important steroids are a
group of compounds having vitamin D activity.
Waxes
Waxes are water-insoluble,
solid esters of higher fatty acids with long-chain monohydroxylic fatty
alcohols or with sterols. They are soft and pliable when warm but hard when
cold. Waxes are found as protective coatings on skin, fur, and feathers, on
leaves and fruits of higher plants, and on the exoskeleton of many insects. The
major components of beeswax are palmitic acid esters of long-chain fatty
alcohols with 26 to 34 carbon atoms. Lanolin,
or wool fat, is a mixture of fatty acid esters of the sterols lanosterol and
agnosterol.
Waxes are used to
coat and protect things in nature. Bees make wax. Your ears make wax. Plant
leaves even have wax on the outside of their leaves. It can be used for
structures such as the bees' honeycombs. Waxes can also be used for protection.
Plants use wax to stop evaporation
of water from their leaves.
Prostaglandins Thromboxanes & Leukotrienes
The members of this group of structurally
related natural hormones have an extraordinary range of biological effects.
They can lower gastric secretions, stimulate uterine contractions, lower blood
pressure, influence blood clotting and induce asthma-like allergic responses.
Because their genesis in body tissues is tied to the metabolism of the
essential fatty acid arachadonic acid (5,8,11,14-eicosatetraenoic acid) they
are classified as eicosanoids. Many
properties of the common drug asprin result from its effect on the cascade of
reactions associated with these hormones.
 The metabolic pathways by which arachidonic acid is converted to the
various eicosanoids are complex and will not be discussed here. A rough outline
of some of the transformations that take place is provided below. It is helpful
to view arachadonic acid in the coiled conformation shown in the shaded box.
The metabolic pathways by which arachidonic acid is converted to the
various eicosanoids are complex and will not be discussed here. A rough outline
of some of the transformations that take place is provided below. It is helpful
to view arachadonic acid in the coiled conformation shown in the shaded box.
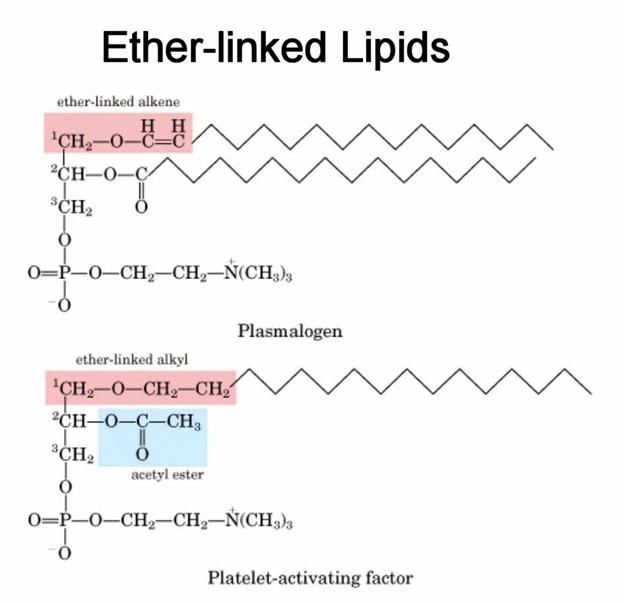
Phospholipids
http://www.youtube.com/watch?v=PoolWjqoyO0
Glycerophospholipids
(phosphoglycerides)
The basic structure of phospolipids is very similar to that of the
triacylglycerides except that C-3 (sn3)of
the glycerol backbone is esterified to phosphoric acid. The building block of
the phospholipids is phosphatidic acid which results when the X substitution in
the basic structure shown in the Figure below is a hydrogen atom. Substitutions
include ethanolamine (phosphatidylethanolamine), choline (phosphatidylcholine,
also called lecithins), serine (phosphatidylserine), glycerol
(phosphatidylglycerol), myo-inositol
(phosphatidylinositol, these compounds can have a variety in the numbers of
inositol alcohols that are phosphorylated generating
polyphosphatidylinositols), and phosphatidylglycerol.
Phosphoglycerides are characteristic
major components of cell membranes; only very small amounts of
phosphoglycerides occur elsewhere in cells.
Phospholipids are made from glycerol, two fatty acids, and (in place of
the third fatty acid) a phosphate
group with some other
molecule attached to its other end. The hydrocarbon tails of the fatty acids
are still hydrophobic, but the phosphate group end of the molecule is
hydrophilic because of the oxygens with all of their pairs of unshared
electrons. This means that phospholipids are soluble in both water and oil.
|
|
An emulsifying
agent is a substance which is soluble in both oil and water, thus enabling
the two to mix. A “famous” phospholipid is lecithin which is found in egg yolk and soybeans.
Egg yolk is mostly water but has a lot of lipids, especially cholesterol, which
are needed by the developing chick. Lecithin is used to emulsify the lipids and hold them in the water as an emulsion. Lecithin is the basis of the
classic emulsion known as mayonnaise.
http://www.youtube.com/watch?v=7k2KAfRsZ4Q&feature=related
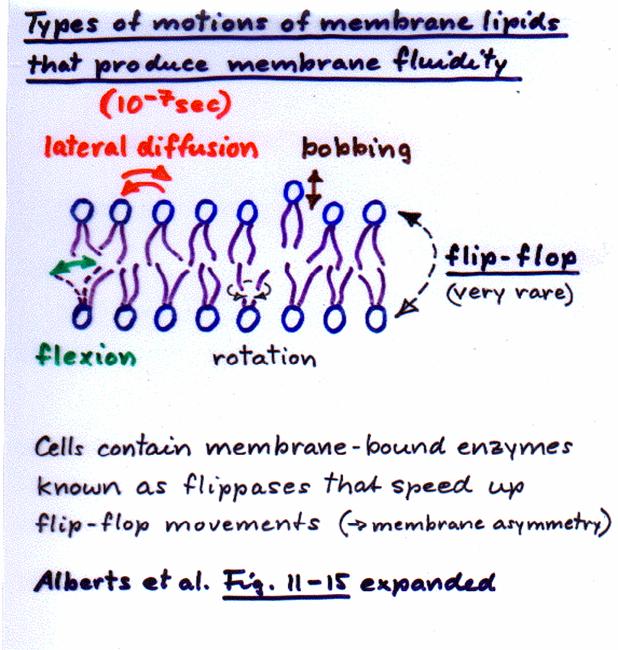
Our cell
membranes are made mostly of phospholipids arranged in a double
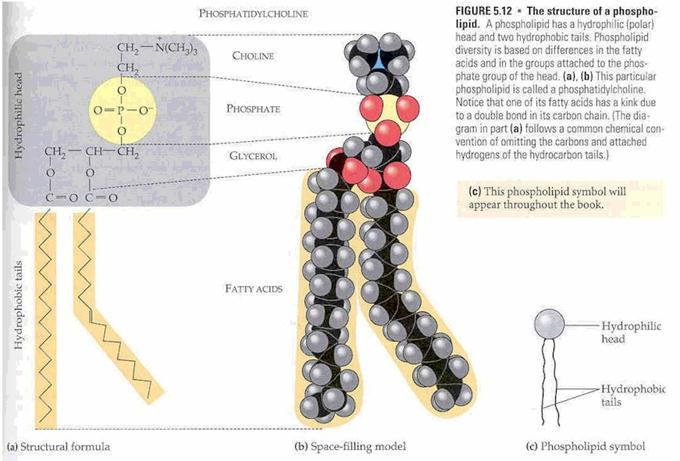
layer with the tails from both layers
“inside” (facing toward each other) and the heads facing “out” (toward the
watery environment) on both surfaces.
In phosphoglycerides one of
the primary hydroxyl groups of glycerol is esterified to phosphoric acid; the
other hydroxyl groups are esterified to fatty acids. The parent compound of
the series is thus the phosphoric ester of glycerol.
Because
phosphoglycerides possess a polar head in addition to their nonpolar
hydrocarbon tails, they are called amphipathic
or polar lipids. The different types
of phosphoglycerides differ in the size, shape, and electric charge of their
polar head groups.
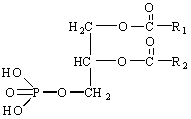 The
parent compound of the phosphoglycerides is phosphatidic
acid, which contains no polar alcohol head group. It occurs in only very
small amounts in cells, but it is an important intermediate in the biosynthesis
of the phosphoglycerides.
The
parent compound of the phosphoglycerides is phosphatidic
acid, which contains no polar alcohol head group. It occurs in only very
small amounts in cells, but it is an important intermediate in the biosynthesis
of the phosphoglycerides.
posphatidic acid
The most abundant phosphoglycerides
in higher plants and animals are phosphatidylethanoamme
and phosphatidylchohne, which contain
as head groups the amino alcohols ethanoiamine
and choline, respectively.
(The new names recommended for these phosphoglycerides are ethanolamine
phosphoglyceride and choline phosphoglyceride, but they have not yet
gained wide use. The old trivial names are cephalin
and lecithin, respectively.) These
two phosphoglycerides are major components of most animal cell membranes.
In phosphqtidylserine, the hydroxyl
group of the amino acid L-serine is esterified to the phosphoric acid.
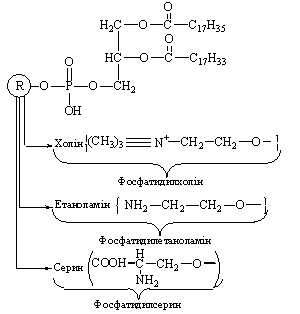
Closely related to
phosphatidylglycerol is the more complex lipid cardiolipin, also called diphosphatidylglycerol,
which consists of a molecule of phosphatidylglycerol in which the 3'-hydroxyl
group of the second glycerol moiety is esterified to the phosphate group of a
molecule of phosphatidic acid. The backbone of cardiolipin thus consists of three molecules of glycerol joined by
two phosphodiester bridges; the two hydroxyl groups of both external glycerol
molecules are esterified with fatty acids. Cardiolipin
is present in large amounts in the inner membrane of mitochondria; it was first
isolated from heart muscle, in which mitochondria are abundant.
http://www.youtube.com/watch?v=kOTRNFZHmTI&feature=related
Lipid
Soluble Vitamins
The essential dietary
substances called vitamins are
commonly classified as "water soluble" or "fat soluble". Water
soluble vitamins, such as vitamin C, are rapidly eliminated from the body and
their dietary levels need to be relatively high. The recommended daily
allotment (RDA) of vitamin C is 100 mg, and amounts as large as 2 to 3 g are
taken by many people without adverse effects. The lipid soluble vitamins, shown
in the diagram below, are not as easily eliminated and may accumulate to toxic
levels if consumed in large quantity. The RDA for these vitamins are:
Vitamin A 800 μg ( upper
limit ca. 3000 μg)
Vitamin D 5 to 10 μg ( upper
limit ca. 2000 μg)
Vitamin E 15 mg ( upper limit ca. 1 g)
Vitamin K 110 μg ( upper
limit not specified)
From this data it is clear that vitamins A and D, while essential to
good health in proper amounts, can be very toxic. Vitamin D, for example, is
used as a rat poison, and in equal weight is more than 100 times as poisonous
as sodium cyanide. From the structures shown here, it should be clear that
these compounds have more than a solubility connection with lipids. Vitamins A
is a terpene, and vitamins E and K have long terpene chains attached to an
aromatic moiety. The structure of vitamin D can be described as a steroid in
which ring B is cut open and the remaining three rings remain unchanged. The
precursors of vitamins A and D have been identified as the tetraterpene
beta-carotene and the steroid ergosterol, respectively.
Properties of
phosphoglycerides
Phosphoglycerides are soluble in most nonpolar solvents
containing some water and are best extracted from cells and tissues with
chloroform-methanol mixtures. When
phosphoglycerides are placed in water, they appear to dissolve, but only very
minute amounts go into true solution; most of the "dissolved" lipid
is in the form of micelles.
Phosphoglycerides have variations in the size, shape, polarity, and
electric charge and it plays a significant role in the structure of various
types of cell membranes.
Phosphoglycerides can be hydrolyzed
by specific phospholipases, which
have become important tools in the determination of phosphoglyceride structure.
Phospholipase A1 specifically removes the fatty acid from the 1 position and
phospholipase A2 from the 2 position. Removal of one fatty acid molecule from
a phosphoglyceride yields a lysophosphoglyceride,
e.g., lysophosphatidyl-ethanolamine. Lysophosphoglycerides are
intermediates in phosphoglyceride metabolism but are found in cells or tissues
in only very small amounts; in high concentrations they are toxic and injurious
to membranes. Phospholipase B can bring about successive removal of the two
fatty acids of phosphoglycerides. Phospholipase C hydrolyzes the bond between
phosphoric acid and glycerol, while phospholipase D removes the polar head
group to leave a phosphatidic acid.
Sphingophospholipids
Sphingolipids are composed of a backbone of sphingosine which is derived
itself from glycerol. Sphingosine is N-acetylated by a variety of fatty acids
generating a family of molecules referred to as ceramides. Sphingolipids
predominate in the myelin sheath of nerve fibers. Sphingomyelin is an abundant
sphingolipid generated by transfer of the phosphocholine moiety of
phosphatidylcholine to a ceramide, thus sphingomyelin is a unique form of a
phospholipid.
The other
major class of sphingolipids (besides the sphingomyelins) are the glycosphingolipids
generated by substitution of carbohydrates to the sn1 carbon of the glycerol backbone of a ceramide. There are 4
major classes of glycosphingolipids:
n
Cerebrosides: contain a single moiety, principally galactose.
n
Sulfatides: sulfuric acid esters of galactocerebrosides.
n
Globosides: contain 2 or more sugars.
n
Gangliosides: similar to globosides except also contain sialic acid.
Sphingolipids
Sphingophospholipids, complex
lipids containing as their backbone sphingosine, are important membrane components in both plant and animal
cells. They are present in especially large amounts in brain and nerve tissue.
Only trace amounts of sphingophospholipids are found in depot fats. All
sphingophospholipids contain four characteristic building-block components: one
molecule of a fatty acid, one molecule of sphingosine, phosphoric acid and a polar
head group, which in some sphingolipids is very large and complex.
The most abundant sphingophospholipids in the tissues of
higher animals are sphingomyelins, which contain phosphorylethanolamine or
phosphorylcholine as their polar head groups, esterified to the 1-hydroxyl
group of ceramide. Sphingomyelins have physical properties very similar to
those of phosphatidylethanolamine and phosphatidylcholine.
Glycolipids
Glycosylglycerols
Glycosyldiqcylglycerols contain a sugar in glycosidic linkage with the unesterified 3-hydroxyl
group of diacylglycerols. A common example is galactosyldiacylglycerol, found in higher plants
and also in neural tissue of vertebrates.
Glycosphingolipids
Neutral glycosphingolipids
This class of glycolipids
contains one or more neutral sugar residues as their polar head groups and thus
has no electric charge; they are called neutral glycosphingolipids. The
simplest of these are the cerebrosides,
which contain as their polar head group a monosaccharide bound in
beta-glycosidic linkage to the hydroxyl group of ceramide. The cerebrosides of the brain and nervous system contain
D-galactose and are therefore called galactocerebrosides.
Cerebrosides are also present in much smaller amounts in nonneural tissues of
animals, where, because they usually contain D-glucose instead of D-galactose,
they are called glucocerebrosides.
Sulfate esters of galactocerebrosides
(at the 3 position of the D-galactose) are also present in brain tissue; they
are called sulfotides.
The neutral glycosphingolipids are
important cell-surface components in animal tissues. Their nonpolar tails
presumably penetrate into the lipid bilayer structure of cell membranes,
whereas the polar heads protrude outward from the surface. Some of the neutral
glycosphingolipids are found on the surface of red blood cells and give them
blood-group specificity.
Acidic
glycosphingolipids (gangliosides)
Gangliosides contain in their oligosaccharide
head groups one or more residues of a sialic acid, which gives the polar head
of the gangliosides a net negative charge at pH 7.0. The sialic acid usually
found in human gangliosides is N-acetylneuraminic
acid. Gangliosides are most abundant in the gray matter of the brain, where
they constitute 6 percent of the total lipids, but small amounts are also found
in nonneural tissues.
Function of glycosphingolipids
Although glycosphingolipids are only minor
constituents of membranes, they appear to be extremely important in a number
of specialized functions. Because gangliosides are especially abundant in nerve
endings, it has been suggested that they function in the transmission of nerve
impulses across synapses. They are also believed to be present at receptor
sites for acetylcholine and other neurotransmitter substances. Some of the
cell-surface glycosphingolipids are concerned not only in blood-group
specificity but also in organ and tissue specificity. These complex lipids are
also involved in tissue immunity and in cell-cell recognition sites fundamental
to the development and structure of tissues. Cancer cells, for example, have
characteristic glycosphingolipids different from those in normal cells.
The most important
derivatives of lipids
The lipids discussed up to
this point contain fatty acids as building blocks, which can be released on
alkaline hydrolysis. The simple lipids contain no fatty acids. They occur in
smaller amounts in cells and tissues than the complex lipids, but they include
many substances having profound biological activity—vitamins, hormones, and
other highly specialized fat-soluble biomolecules.
Prostaglandins are a family of
fatty acid derivatives which have a variety of potent biological activities of
a hormonal or regulatory nature. Prostaglandins function as regulators of
metabolism in a number of tissues and in a number of ways.
All the natural prostaglandins
are biologically derived by cyclization of 20-carbon unsaturated fatty acids,
such as arachidonic acid, which is formed from the essential fatty acid
linoleic acid. The prostaglandins differ from each other with respect to their
biological activity, although all show at least some activity in lowering
blood pressure and inducing smooth muscle to contract. Some, like PGE2,
antagonize the action of certain hormones. PGE2 and PGE2a may find clinical use in inducing labor and bringing about therapeutic
abortion.
Digestion of fats
By
far the most common of the diet are the neutral fats, also known as triglycerides, each molecule of which is
composed of a glycerol nucleus and three fatty acids, as illustrated. Neutral
fat is found in food of both animal and and plant origin. In the usual diet are
also small quantities of phospholipids, cholesterol, and cholesterol esters.
Digestion of fats in the intestine. A small amount of short chain triglycerides is
digested in the stomach by gastric
lipase.
Emulsification of fat by bile acids. The first in fat digestion is to break the fat
globules into s sizes so that the water-soluble digestive enzymes act on the
globule surfaces. This process is called emulsification
of the
fat, and it is achieved under 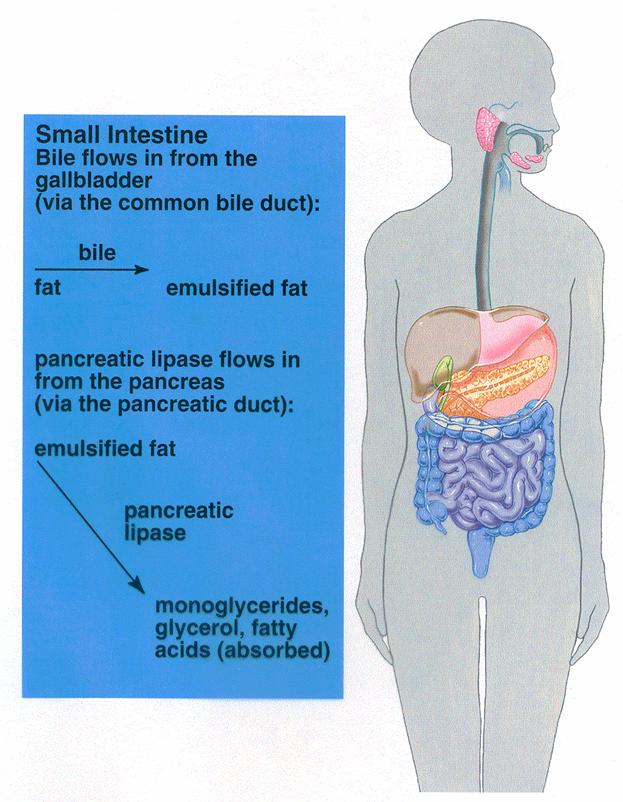
the
presence of bile acids. Bile contain
a large quantity of bile salts,
mainly in the form of ionized sodium salts.
The carboxyl and other parts of the
bile salt molecule are highly soluble in water, whereas most of the sterol
portion of the bile is highly soluble in fat. Therefore, the fat-soluble
portion of the bile salt dissolves in the surface layer of the fat globule and
polar portion of the bile salt is soluble in the surrounding fluids. This
effect decreases the interfacial tension of the fat. When the interfacial
tension of a globule is low, globule is broken up into many minute particles.
The total surface area of the particles in the intestinal contents is inversely
proportional to the diameters of the particles. The lipases are water-soluble
compounds and can act on the fat globules only on their surfaces. Consequently,
it can be readily understood how important detergent function of bile salts is
for the digestion of fats.
Digestion of fats by pancreatic
lipase. The most
important enzyme for the digestion of fats is pancreatic lipase in the pancreatic juice. However, the cells of
the small intestine also contain a minute quantity of lipase known as enteric lipase. Both liiese act alike to cause hydrolysis of fat.
Products of fat digestion. Most of the triglycerides of the
diet are split into free fatty acids and monoglycerides.
Role of bile salts in
accelerating fat digestion — formation of micelles. The hydrolysis of triglycerides
highly reversible process; therefore, accumulation of monoglycerides and free
fatty acids very quickly blocks further digestion. The bile salts play an important role in removing the
monoglycerides and free fatty acids from the vicinity of the digesting fat globules almost as rapidly
as these end-products of digestion are formed. This occurs in the following way: bile salts have
the propensity to form micelles, which are small spherical globules
composed of 20 to 40 molecules of bile salt. These develop because each bile salt
molecule is composed of a sterol nucleus, most of which is highly fat-soluble,
and a polar group that is highly water-soluble. The sterol nuclei of the 20 to 40 bile
salt molecules of the micelle aggregate together to form a small fat globule in
the middle of the micelle. This aggregation causes the polar groups to project
outward to cover the surface of the micelle During triglyceride digestion, as rapidly
as the monoglycerides and free fatty acids are formed they become dissolved in
the fatty portion of the micelles, which immediately reduces these end-products
of digestion in the vicinity of the digesting fat globules. The bile salt
micelles also act as a transport medium to carry the monoglycerides and the
free fatty acids, both of which would otherwise be relatively insoluble, to the
brush borders of the epithelial cells. There the monoglycerides and free fatty
acids are absorbed. On delivery of these substances to the brush border,
the bile salts are again released 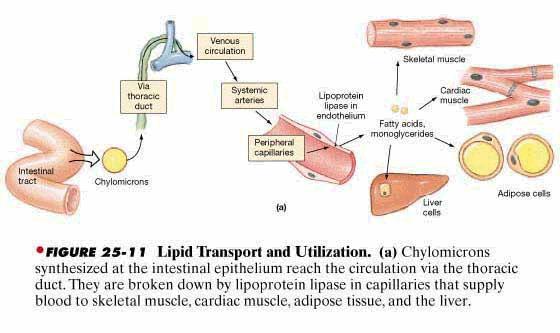
back into the chyme to be
used again and again for this "ferrying" process.
Digestion of Cholesterol Esters and Phospholipids. Most of the cholesterol in the diet is in the form of cholesterol esters, which are combinations of free cholesterol and one molecule of fatty acid. And phospholipids also contain fatty acid chains within their molecules. Both the cholesterol esters and the phospholipids are hydrolyzed by lipases in the pancreatic secretion that free the fatty acids — the enzyme cholesterol ester hydrolase to hydrolyze the cholesterol ester and phospholipase A to hydrolyze the phospholipid.
The bile
salt micelles play identically the same role in "ferrying" free
cholesterol as they play in "ferrying" monoglycerides and free fatty
acids. Indeed,
this role of the bile salt micelles is absolutely essential to the absorption
of cholesterol because essentially no cholesterol is absorbed without the
presence of bile salts. On the other hand, as much as 60 per cent of the
triglycerides can be digested and absorbed even in the absence of bile salts.
Absorption of fats
Monoglycerides and fatty
acids - both of digestive end-products - become dissolved in the lipid portion
of the micelles. Because of the molecular dimension of these micelles, only 2.5
nanometers, and also because of their highly charged, they are soluble in the
chyme. Micelles
contact with the surfaces of the brush border even penetrating into the
recesses , agitating microvilli. 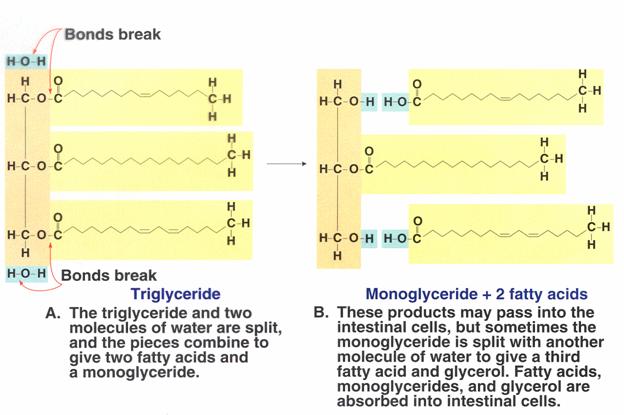
The micelles then diffuse back through the chyme and absorb still more monoglycerides and fatty acids, and similarly transport these also to the epithelial cells. Thus, the bile acids perform a "ferrying" function, which is highly important for fat absorption. In the presence of an abundance of bile acids, approximately 97 per cent of the fat is absorbed; in the absence of bile acids, only 50 to 60 per cent is normally absorbed.
The mechanism for absorption of the monoglycerides
and fatty acids through the brush border is based entirely on the fact that
both these substances are highly lipid-soluble. Therefore, they become dissolved in the
membrane and simply diffuse to the interior of the cell. The undigested triglycerides and the
diglycerides are both also highly soluble in the lipid membrane of the
epithelial cell. However, only small quantities of these are normally
absorbed because the bile acid micelles will not dissolve either triglycerides
or diglycerides and therefore will not ferry them to the epithelial membrane.
After entering the epithelial cell, the fatty acids and monoglycerides
are taken up by the smooth endoplasmic reticulum, and here they are mainly
recombined to form new triglycerides. However, a few of the monoglycerides are
further digested into glycerol and fatty acids by an epithelial cell lipase. Then, the free
fatty acids are reconstituted by the smooth endoplasmic reticulum into
triglycerides. Most of the glycerol that is utilized for this
purpose is synthesized de novo from alpha-glycerophosphate, this synthesis
requiring both energy from ATP and a complex of enzymes to catalyze the
reactions. Once formed, the triglycerides aggregate within the endoplasmic reticulum into
globules along with absorbed cholesterol, absorbed phospholipids, and small
amounts of newly synthesized cholesterol and phospholipids. The phospholipids
arrange themselves in these globules with the fatty portion of the phospholipid
toward the center and the polar portions located on the surface. This
provides an electrically charged surface that makes these globules miscible
with the fluids of the cell.
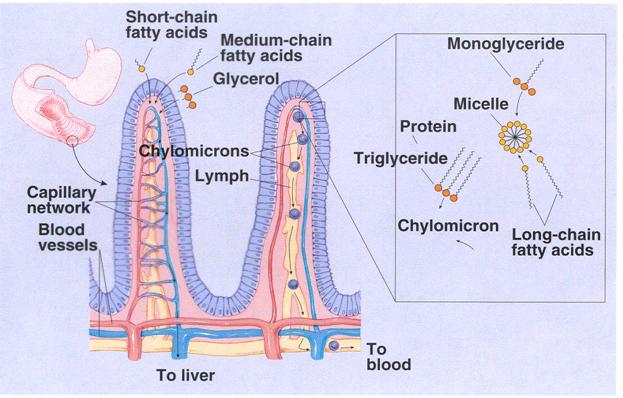
In
addition, small amounts of lipoprotein, also synthesized by the endoplasmic
reticulum, coat part of the surface of each globule. In this form the globule diffuses to the side of the
epithelial cell and is excreted by the process of cellular exocytosis into the space between the cells; from there it passes into the
lymph in the central lacteal of the villus. These globules are then called chylomicrons.
Transport of the Chylomicrons in the Lymph. From the sides of the
epithelial cells the chylomicrons wend their way into the central lac-teals of
the villi and from here are propelled, along with the lymph, by the lymphatic
pump upward through the thoracic duct to be emptied into the great veins of the
neck. Between 80 and 90 per
cent of all fat absorbed from the gut is absorbed in this manner and is transported
to the blood by way of the thoracic
lymph in the form of chylomicrons.
Direct Absorption of fatty acids into the portal blood. Small quantities of
short chain fatty acids, such as those from butterfat, are absorbed directly
into the portal blood rather than being converted into triglycerides and
absorbed into the lymphatics.
The cause
of this difference between short and long chain fatty acid absorption is that
the shorter chain fatty acids are more water-soluble and are not reconverted
into triglycerides by the endoplasmic reticulum. This allows direct diffusion of these fatty acids from the
epithelial cells into the capillary blood of the dlood.
|
|
Catabolism of triacylglycerols
Dietary
acylglycerols undergo hydrolysis in the small intestine by the action of
lipases, e.g., those present in pancreatic juice. Lipase digests the
triacylglycerols to 2-monoglycerols, glycerol and free fatty acids. These
components are absorbed and metabolized in the enterocytes, blood and liver. In
the enterocytes and liver the specific for organism acylglycerols are
synthesized. Then these are accumulated in
adipose tissue and in much
smaller quantity in other
organs.
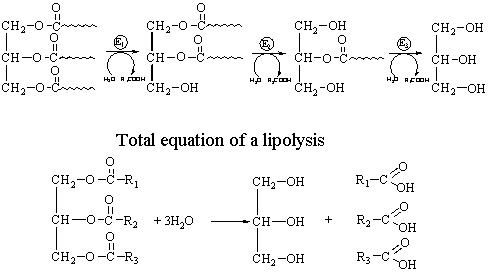
Fermentative hydrolysis of in
adipocytes and other cells is implemented in several stages. Diacylglycerols,
monoacylglycerols, glycerol and free fatty acids are formed in this process:
 OXIDATION OF FATTY ACIDS
OXIDATION OF FATTY ACIDS
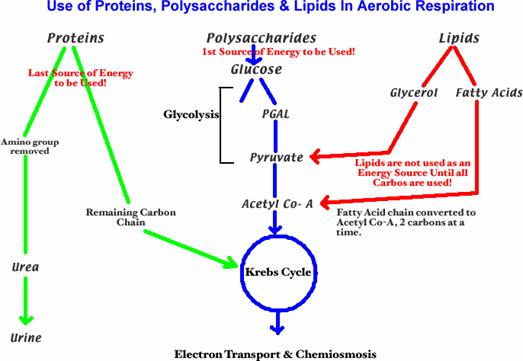
Fatty acids
play an extremely important part as an energy-rich fuel in higher animals and
plants since large amounts can be stored in cells in the form of
triacylglycerols. Triacylglycerols are especially well adapted for this role
because they have a high energy content (about 9 kcal/g) and can be accumulated
in nearly anhydrous form as intracellular fat droplets. In contrast, glycogen
and starch can yield only about 4 kcal/g; moreover, since they are highly
hydrated, they cannot be stored in such
concentrated form. Fatty acids provide up to 40 percent of the total fuel
requirement in man on a normal diet.
http://www.youtube.com/watch?v=3xF_LK9pnL0&feature=related
Sources of Fatty
Acids.
Mammalian tissues normally contain only
vanishingly small amounts of free fatty acids, which are in fact somewhat
toxic. By the action of hormonally controlled
lipases free fatty acids are formed from triacylglycerols in fat or adipose
tissue. The free fatty acids are then released from the tissue, become tightly
bound to serum albumin, and in this form are carried via the blood to other
tissues for oxidation. Fatty acids delivered in this manner are first
enzymatically "activated" in the cytoplasm and then enter the
mitochondria for oxidation.
Long-chain fatty acids are
oxidized to CO2 and H2O in nearly all tissues of
vertebrates except the brain. Some tissues, such as heart muscle, obtain most
of their energy from the oxidation of fatty acids. The mobilization,
distribution, and oxidation of fatty acids are integrated with the utilization
of carbohydrate fuels; both are under complex endocrine regulation.
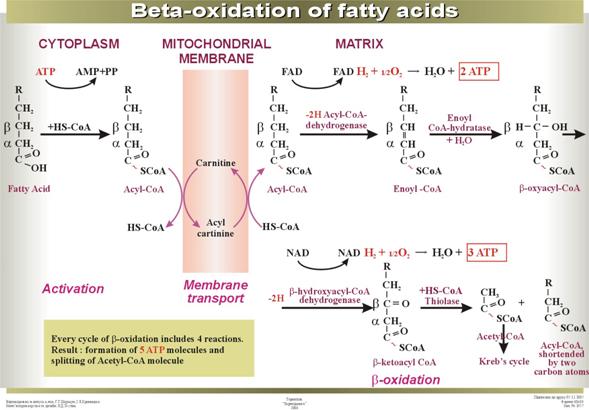
The pathway of fatty acid oxidation.
Knoop
postulated that fatty acids are oxidized by b-oxidation, i.e., oxidation at the b carbon to yield a b-keto acid,
which was assumed to undergo cleavage to form acetic acid and a fatty acid
shorter by two carbon atoms.
Outline of the fatty acid oxidation
cycle.
Before
oxidation, long-chain fatty acids from the cytosol must undergo a rather
complex enzymatic activation, followed by transport across the mitochondrial
membranes into the major compartment. There the fatty acyl group is transferred
to intramitochondrial coenzyme A, yielding a fatty acyl-CoA thioester. The
subsequent oxidation of the fatty acyl-CoA takes place entirely in the
mitochondrial matrix. The fatty
acyl-CoA is dehydrogenated by removal of a pair of hydrogen atoms from the a and b carbon atoms (atoms 2 and 3) to yield the a,b-unsaturated acyl-CoA. This is then enzymatically hydrated to form a b-hydroxyacyl-CoA, which in turn is dehydrogenated in
the next step to yield the b-ketoacyl-CoA. It then undergoes enzymatic cleavage by reaction with a
second molecule of CoA. One product is acetyl-CoA, derived from carbon atoms 1
and 2 of the original fatty acid chain. The other product, a long-chain
saturated fatty acyl-CoA having two fewer carbon atoms than the original fatty
acid, now becomes the substrate for another round of reactions, beginning at the
first dehydrogenation step and ending with the removal of a second two-carbon
fragment as acetyl-CoA. At each passage through this spiral the fatty acid
chain loses a two-carbon fragment as acetyl-CoA. The 16-carbon palmitic acid
thus undergoes a total of seven such cycles, to yield altogether 8 molecules of
acetyl-CoA and 14 pairs of hydrogen atoms. The palmitate must be primed or
activated only once, since at the end of each round the shortened fatty acid
appears as its CoA thioester.
The hydrogen atoms
removed during the dehydrogenation of the fatty acid enter the respiratory
chain; as electrons pass to molecular oxygen via the cytochrome system,
oxidative phosphorylation of ADP to ATP occurs. The acetyl-CoA formed as
product of the fatty acid oxidation system enters the tricarboxylic acid cycle.
Activation and entry of fatty acids into mitochondria.
There are three stages in the entry of fatty acids
into mitochondria from the extramitochondrial cytoplasm: (1) the enzymatic
ATP-driven esterification of the free fatty acid with extramitochondrial CoA to
yield fatty acyl-CoA, a step often referred to as the activation of the fatty
acid, (2) the transfer of the acyl group from the fatty acyl-CoA to the carrier
molecule carnitine, followed by the transport of the acyl carnitine across the
inner membrane, and (3) the transfer of the acyl group from fatty acyl
carnitine to intramitochondrial CoA.
Activation of fatty acids.
At least three different enzymes
catalyze formation of acyl-CoA thioesters, each being specific for a given
range of fatty acid chain length. These enzymes are called acyl-CoA
synthetases. Acetyl-CoA synthetase activates acetic, propionic, and acrylic
acids, medium-chain acyl-CoA synthetase activates fatty acids with 4 to
12 carbon atoms, and long-chain acyI-CoA synthetase activates fatty acids with
12 to 22 or more carbon atoms. The last two enzymes activate both saturated
and unsaturated fatty acids. Otherwise the properties and mechanisms of all
three synthetases, which have been isolated in highly purified form, are nearly
identical. The overall reaction catalyzed by the ATP-linked acyl-CoA
synthetases is:
RCOOH + ATP + CoA–SH Û
RCO—S—CoA + AMP + PP
Fatty
acids acyl-CoA
In this
reaction a thioester linkage is formed between the fatty acid carboxyl group
and the thiol group of CoA; the ATP undergoes pyrophosphate cleavage to yield
AMP and inorganic pyrophosphate.
The acyl-CoA synthetases are found in the outer mitochondrial membrane and in the endoplasmic reticulum.
Transfer
to carnitine.
Long-chain saturated fatty acids have
only a limited ability to cross the inner membrane as CoA
thioesters, but their entry is greatly stimulated by carnitine.
The stimulation of fatty acid
oxidation by carnitine is due to the action of an enzyme carnitine acyltransferase, which catalyzes transfer of the fatty
acyl group from its thioester linkage with CoA to an oxygen-ester linkage with
the hydroxyl group of carnitine. The acyl carnitine ester so formed then passes
through the inner membrane into the matrix, presumably via a specific
transport system.
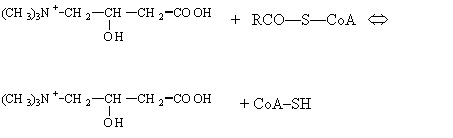
Carnitine Acyl-CoA
![]()
Acyl-carnitine
Transfer to
intramitochondrial CoA.
In the last stage of the entry process the acyl group
is transferred from carnitine to intramitochondrial CoA by the action of a
second type of carnitine acyltransferase
located on the inner surface of the inner membrane:
Acyl carnitine + CoA Û acyl-CoA + carnitine
This complex entry mechanism,
often called the fatty acid shuttle, has the effect of keeping the
extramitochondrial and intramitochondrial pools of CoA and of fatty acids
separated. The intramitochondrial fatty acyl-CoA now becomes the substrate of
the fatty acid oxidation system, which is situated in the inner matrix
compartment.
The first dehydrogenation step in
fatty acid oxidation.
Following
the formation of intramitochondrial acyl-CoA, all subsequent reactions of the
fatty acid oxidation cycle take place in the inner compartment. In the first
step the fatty acyl-CoA thioester undergoes enzymatic dehydrogenation by acyl-CoA dehydrogenase at the a and b carbon atoms (carbons 2 and 3) to form enoyl-CoA as
product. The double bond formed in this reaction has the trans geometrical
configuration. Recall, however, that the double bonds of the unsaturated fatty
acids of natural fats nearly always have the cis configuration.
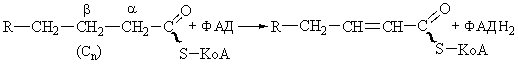
There are four different
acyl-CoA dehydrogenases, each specific for a given range of fatty acid chain
lengths. All contain tightly bound flavin adenine dinucleotide (FAD) as prosthetic
groups. The FAD becomes reduced at the expense of the substrate, a process that
probably occurs through distinct one-electron steps.
The FADH2 of the
reduced acyl-CoA dehydrogenase cannot react directly with oxygen but donates
its electrons to the respiratory chain
via a second flavoprotein, electron-transferring flavoprotein, which in
turn passes the electrons to some carrier of the respiratory chain.
The
hydration step.
The double bond of the enoyl-CoA ester is then hydrated to form 3-hydroxyacyl-CoA by the enzyme enoyl-CoA hydratase.
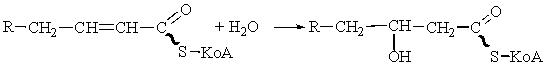
The addition of water across the trans double bond is stereo-specific
and results in the formation of the L-stereoisomer of the 3-hydroxyacyl-CoA.
The
second dehydrogenation step.
In the next
step of the fatty acid oxidation cycle, the 3-hydroxyacyl-CoA is dehydrogenated
to form 3-ketoacyl-CoA) by 3-hydroxyacyl-CoA dehydrogenase. NAD+
is the specific electron acceptor. The reaction is:
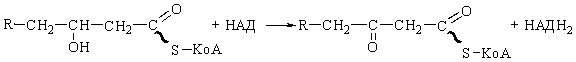
This enzyme
is relatively nonspecific with respect to the length of the fatty acid chain
but is absolutely specific for the l stereoisomer.
The NADH formed in the reaction donates its electron equivalents to the NADH
dehydrogenase of the mitochondrial respiratory chain.
The cleavage step.
In the last step of the fatty
acid oxidation cycle, which is catalyzed by acetyl-CoA
acetyltransferase, more commonly
known as thiolase, the 3-ketoacyl-CoA
undergoes cleavage by interaction with a molecule of free CoA to yield the carboxyl-terminal
two-carbon fragment of the fatty acid as acetyl-CoA. The remaining fatty acid,
now shorter by two carbon atoms, appears as its coenzyme A thioester.
This cleavage reaction, also called a
thiolysis or a thiolytic cleavage, is analogous to hydrolysis. Since the
reaction is highly exergonic, cleavage is favored. There appear to be two
(perhaps three) forms of the enzyme, each specific for different fatty acid
chain lengths.
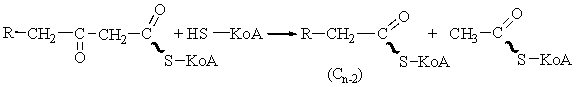
The balance sheet.
We have described one turn of the
fatty acid oxidation cycle, in which one molecule of acetyl-CoA and two pairs
of hydrogen atoms have been removed from the starting long-chain fatty
acyl-CoA. The overall equation for one turn of the cycle, starting from
palmitoyl-CoA, is
Palmitoyl-CoA
+ CoA + FAD+ + NAD+
+ H2O ®
myristoyl-CoA + acetyl-CoA + FADH2
+ NADH2
We
can now write the equation for the seven turns of the cycle required to convert
one molecule of palmitoyl-CoA into eight molecules of acetyl-CoA:
Palmitoyl-CoA
+ 7CoA + 7FAD+ + 7NAD+ + 7H2O ®
8 acetyl-CoA +
7FADH2 + 7NADH2 + 7H+
Each molecule of FADH2
donates a pair of electron equivalents to the respiratory chain at the level
of coenzyme Q; thus two molecules of ATP are generated during the ensuing
electron transport to oxygen. Similarly, oxidation of each molecule of NADH2
by the respiratory chain results in formation of three molecules of ATP. Hence, a total of five molecules of ATP is
formed by oxidative phosphorylation per molecule of acetyl-CoA cleaved.
The seven turns of the cycle required to
convert one molecule of palmitoyl-CoA rsults in the formation of 5 x 7 = 35
ATP.
The eight molecules of acetyl-CoA formed in the
fatty acid cycle may now enter the tricarboxylic acid cycle. The degradation of
1 molecule of acetyl-CoA in tricarboxylic acid cycle results in the formation
of 12 molecules of ATP. 8 molecules of acetyl-CoA give 96 molecules of ATP.
Thus, the total output of energy in full
cleavage of 1 molecule of palmitoyl-CoA is: 35 + 96 = 131 molecules of ATP.
Since one molecule of ATP is
in effect utilized to form palmitoyl-CoA from palmitate, the net yield of ATP
per molecule of palmitate is 130.
Oxidation of unsaturated fatty acids.
Unsaturated
fatty acids, such as oleic acid, are oxidized by the same general pathway as
saturated fatty acids, but two special problems arise. The double bonds of
naturally occurring unsaturated fatty acids are in the cis configuration,
whereas the unsaturated acyl-CoA intermediates in the oxidation of saturated
fatty acids are trans, as we have seen. Moreover, the double bonds of most
unsaturated fatty acids occur at such positions in the carbon chain that successive
removal of two-carbon fragments from the carboxyl end yields a D3-unsaturated fatty acyl-CoA rather than the D2 fatty acyl-CoA serving as the normal
intermediate in the fatty acid cycle.
These problems have been resolved
with the discovery of an auxiliary enzyme, enoyl-CoA isomerase, which
catalyzes a reversible shift of the double bond from the D3-cis to the D2-trans configuration. The resulting
D2-trans-unsaturated
fatty acyl-CoA is the normal substrate for the next enzyme of the fatty acid
oxidation sequence, enoyl-CoA hydratase,
which hydrates it to form L-3-hydroxyacyl-CoA. The complete oxidation of
oleyl-CoA to nine acetyl-CoA units by the fatty acid oxidation cycle thus
requires an extra enzymatic step catalyzed by the enoyl-CoA isomerase, in addition to those steps required in the
oxidation of saturated fatty acids.
Polyunsaturated fatty acids, such as
linoleic acid, require a second auxiliary enzyme to complete their oxidation,
since they contain two or more cis
double bonds. When three successive acetyl-CoA units are removed from
linoleyl-CoA, a D3-cis double
bond remains, as in the case of oleyl-CoA. This is then transformed by the
enoyl-CoA isomerase described above to the D2-trans isomer. This undergoes the usual reactions,
with loss of two acetyl-CoA's, leaving an eight-carbon D2-unsaturated acid. Note,
however that the double bond of the latter is in the cis configuration. Although
the D2-cis double
bond can be hydrated by enoyl-CoA hydratase, the product is the D stereoisomer
of a 3-hydroxyacyl-CoA, not the L stereoisomer normally formed during oxidation
of saturated fatty acids. Utilization of the d
stereoisomer requires a second auxiliary enzyme, 3-hydroxyacyl-CoA
epimerase, which catalyzes epi-merization at carbon atom 3 to yield the l isomer. The product of this
reversible reaction is then oxidized by the L-specific 3-hydroxyacyl-CoA
dehydrogenase and cleaved by thiolase to complete the oxidation cycle. The
remaining six-carbon saturated fatty acyl-CoA derived from linoleic acid can
now be oxidized to three molecules of acetyl-CoA. These two auxiliary enzymes
of the fatty acid oxidation cycle make possible the complete oxidation of all
the common unsaturated fatty acids found in naturally occurring lipids. The
number of ATP molecules yielded during the complete oxidation of an unsaturated
fatty acid is somewhat lower than for the corresponding saturated fatty acid
since unsaturated fatty acids have fewer hydrogen atoms and thus fewer
electrons to be transferred via the respiratory chain to oxygen.
Oxidation of odd-carbon fatty acids and the
fate of propionyl-CoA
Odd-carbon fatty acids, which are
rare but do occur in some marine organisms, can also be oxidized in the fatty
acid oxidation cycle. Successive acetyl-CoA residues are removed until the
terminal three-carbon residue pro-pionyl-CoA is reached. This compound is also
formed in the oxidative degradation of the amino acids valine and isoleucine.
Propionyl-CoA undergoes enzymatic carboxylation in an ATP-dependent process to
form Ds-methylmaionyl-CoA, a reaction catalyzed by propionyl-CoA
corboxylase. This enzyme contains biotin as its prosthetic group. In the next
step Ds-methylmalonyl-CoA undergoes enzymatic epimerization to LR-methylmalonyl-CoA,
by action of methyimaionyl-CoA racemase. In the next reaction step, catalyzed
by methylmalonyl-CoA mutase, LR-methylmalonyl-CoA
is isomerized to succinyl-CoA, which may then undergo deacylation by reversal
of the succinyl-CoA synthetase reaction
to yield free succinate, an intermediate of the tricarboxylic acid
cycle.
Methylmalonyl-CoA mutase requires as
cofactor coenzyme B12.
Study of this intramolecular reaction with isotope tracers has revealed that
it takes place by the migration of the entire —CO—S—CoA group from carbon atom
2 of methylmalonyl-CoA to the methyl carbon atom in exchange for a hydrogen
atom.
Patients suffering from pernicious
anemia, who are deficient in vitamin B12 because of their lack of
intrinsic factor, excrete large amounts of methylmalonic acid and its precursor
propionic acid in the urine, showing that in such patients the coenzyme B12-dependent
methylmalonyl-CoA mutase reaction is defective.
OXIDATION OF GLYCEROL
Glycerol formed in cleavage of
tryacylglycerols enter catabolism or use for biosynthesis of glycerides again.
Before including of glycerol in metabolism it is activated by ATP to
glycerol-3-phosphate by action of glycerol
phosphokinase:
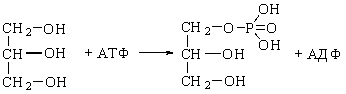
Glycerol Glycerol-3-phosphate
Glycerol-3-phosphate is
oxidized by glycerophosphate
dehydrogenase and glyceroaldehyde-3-phosphate is produced:
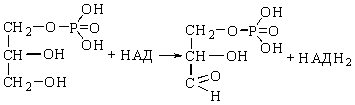
Glycerol-3-phosphate Glyceroaldehyde-3-phosphate
Glyceroaldehyde-3-phosphate is
the central metabolite of glycolysis.
The biosynthesis of lipids is a prominent
metabolic process in most organisms. Because of the limited capacity of higher
animals to store polysaccharides, glucose ingested in excess of immediate
energy needs and storage capacity is converted by glycolysis into pyruvate and
then acetyl-CoA, from which fatty acids are synthesized. These in turn are
converted into triacylglycerols, which have a much higher energy content than
polysaccharides and may be stored in very large amounts in adipose or fat
tissues. Triacylglycerols are also stored in the seeds and fruits of many
plants.
The formation of the various phospholipids and sphingolipids
of cell membranes is also an important biosynthetic process. These complex
lipids undergo continuous metabolic turnover in most cells.
Biosynthesis
of saturated fatty acids
The biosynthesis of saturated fatty acids from their ultimate precursor acetyl-CoA occurs in all organisms but is particularly prominent in the liver, adipose tissues, and mammary glands of higher animals. It is brought about by a process that differs significantly from the opposed process of fatty acid oxidation. In the first place total biosynthesis of fatty acids occurs in the cytosol, whereas fatty acid oxidation occurs in the mitochondria. Second, the presence of citrate is necessary for maximal rates of synthesis of fatty acids, whereas it is not required in fatty acid oxidation. Perhaps the most unexpected difference is that CO2 is essential for fatty acid synthesis in cell extracts, although isotopic CO2 is not itself incorporated into the newly synthesized fatty acids. These and many other observations have revealed that fatty acid synthesis from acetyl-CoA takes place with an entirely different set of enzymes from those employed in fatty acid oxidation.
In the overall reaction of fatty acid
synthesis, which is catalyzed by a complex multienzyme system in the cytosol,
the fatty-acid synthetase complex,
acetyl-CoA derived from carbohydrate or amino acid sources is the ultimate
precursor of all the carbon atoms of the fatty acid chain. However, of the
eight acetyl units required for biosynthesis of palmitic acid, only one is
provided by acetyl-CoA; the other seven arrive in the form of malonyl-CoA,
formed from acetyl-CoA and HCO3- in a carboxylation
reaction. One acetyl residue and seven malonyl residues undergo successive
condensation steps, with release of seven molecules of CO2, to form
palmitic acid; the reducing power is furnished by NADPH:
Acetyl-CoA
+ 7 malonyl-CoA + 14NADPH + 14H+ ®
CH3(CH2)14COOH + 7CO2 + 8CoA
+ 14NADP+ + 6H2O
Palmitic acid
The
single molecule of acetyl-CoA required in the process serves as a primer, or
starter; the two carbon atoms of its acetyl group become the two terminal
carbon atoms (15 and 16) of the palmitic acid formed. Chain growth during fatty
acid synthesis thus starts at the carboxyl group of acetyl-CoA and proceeds by
successive addition of acetyl residues at the carboxyl end of the growing
chain. Each successive acetyl residue is derived from two of the three carbon
atoms of a malonic acid residue entering the system in the form of malonyl-CoA;
the third carbon atom of malonic acid, i.e., that of the unesterified carboxyl
group, is lost as CO2. The final product is a molecule of palmitic
acid.
A distinctive feature of the
mechanism of fatty acid biosynthesis is that the acyl intermediates in the
process of chain lengthening are thio esters, not of CoA, as in fatty acid
oxidation, but of a low-molecular-weight conjugated protein called acyl carrier protein (ACP). This protein
can form a complex or complexes with the six other enzyme proteins required for
the complete synthesis of palmitic acid. In most eukariotic cells all seven
proteins of the fatty acid synthetase complex are associated in a multienzymes
cluster.
In
most organisms the end product of the fatty-acid synthetase system is palmitic
acid, the precursor of all other higher saturated fatty acids and of all
unsaturated fatty acids.
The carbon
source for fatty acid synthesis
The ultimate source of all the carbon
atoms of fatty acids is acetyl-CoA, formed in the mitochondria by the oxidative
decarboxylation of pyruvate, the oxidative degradation of some of the amino
acids, or by the b-oxidation of long-chain fatty acids.
Acetyl-CoA itself cannot pass
out of the mitochondria into the cytosol; however, its acetyl group is
transferred through the membrane in other chemical forms. Citrate, formed in
mitochondria from acetyl-CoA and oxaloacetate, may pass through the
mitochondrial membrane to the cytoplasm via the tricarboxylate transport
system. In the cytosol acetyl-CoA is regenerated from citrate by ATP-citrate lyase, also called citrate cleavage enzyme, which catalyzes
the reaction:
Citrate + ATP + CoA ® acetyl-CoA + ADP + P + oxaloacetate
In a second pathway the acetyl
group of acetyl-CoA is enzymatically transferred to carnitine, which acts as a
carrier of fatty acids into mitochondria preparatory to their oxidation.
Acetylcarnitine passes from the mitochondrial matrix through the mitochondrial
membrane into the cytosol; acetyl-CoA is then regenerated by transfer of the
acetyl group from acetylcarnitine to cytosol CoA.
Formation of malonyl-CoA:
acetyl-CoA carboxylase
Before the acetyl groups of
acetyl-CoA can be utilized by the fatty-acid synthetase complex, an important preparatory
reaction must take place to convert acetyl-CoA into malonyl-CoA, the immediate
precursor of 14 of the 16 carbon atoms of palmitic acid. Malonyl-CoA is formed
from acetyl-CoA and bicarbonate in the cytosol by the action of acetyl-CoA carboxylase, a complex enzyme
that catalyzes the reaction:

Acetyl-CoA
Malonyl-CoA
The carbon atom of the CO2 becomes
the distal or free carboxyl carbon of malonyl-CoA. However, the above equation give
only the overall reaction, the sum of at least three intermediate reactions.
Acetyl-CoA carboxylase contains biotin as its prosthetic group. The
carboxyl group of biotin is bound in amide linkage to the e-amino group of a specific lysine residue of a subunit of the enzyme.
The covalently bound biotin serves as an intermediate carrier of a molecule of
CO2.
The
reaction catalyzed by acetyl-CoA carboxylase, an allosteric enzyme, is the
primary regulatory, or rate-limiting, step in the biosynthesis of fatty acids.
Acetyl-CoA carboxylase is virtually inactive in the absence of its positive
modulators citrate or isocitrate. The striking allosteric stimulation of this
enzyme by citrate accounts for the fact that citrate is required for fatty
acid synthesis in cell extracts without being used as a precursor.
Acetyl-CoA
carboxylase occurs in both an inactive monomeric form and an active polymeric
form. As it occurs in the avian liver, the inactive enzyme monomer has a
molecular weight of 410,000 and contains one binding site for CO2 (that
is, one biotin prosthetic group), one binding site for acetyl-CoA, and one for
citrate. Citrate shifts the equilibrium between the inactive monomer and the
active polymer, to favor the latter.
Polymeric
acetyl-CoA carboxylase of animal tissues consists of long filaments of enzyme
monomers; each monomer unit contains a molecule of bound citrate. The length of
the polymeric form varies, but on the average each filament contains about 20
monomer units, has a particle weight of some 8 megadaltons, and is about 400 nm
long. Such filaments have been studied in the electron microscope and have
actually been observed in the cytoplasm of adipose cells.
The acetyl-CoA carboxylase
reaction is complex. In fact, the monomeric unit of the enzyme contains four
different subunits. The sequence of reactions in the formation of malonyl-CoA
has been deduced from study of the four subunits of the monomer. One of these
subunits, biotin carboxylase (BC),
catalyzes the first step of the overall reaction, namely, the carboxylation of
the biotin residue covalently bound to the second subunit, which is called biotin
carboxyl-carrier protein (BCCP). The second step in the overall reaction is
catalyzed by the third type of subunit, called carboxyl transferase (CT). In these reactions the biotin residue of
the carboxyl carrier protein serves as a swinging arm to transfer the
bicarbonate ion from the biotin carboxylase subunit to the acetyl-CoA bound to
the active site of the carboxyltransferase subunit. The change from the
inactive monomeric form of acetyl-CoA carboxylase to the polymeric, active form
of the enzyme occurs when citrate is bound to the fourth subunit of each
monomeric unit.
The
reactions of the fatty-acid synthetase system
http://www.youtube.com/watch?v=5HF9itMaOKo
http://www.youtube.com/watch?v=NDjffxsKd6c&feature=related
http://www.youtube.com/watch?v=kOTRNFZHmTI&feature=related
Once malonyl-CoA has been generated from acetyl-CoA
by the action of the rather complex acetyl-CoA carboxylase reaction, the
ensuing reactions of fatty acid synthesis occur in a sequence of six successive
steps catalyzed by the six enzymes of the fatty-acid synthetase system. The
seventh protein of this system, which has no enzymatic activity itself, is acyl carrier
protein, to which the
growing fatty acid chain is covalently attached.
Acyl carrier protein (ACP)
Acyl carrier protein,
universally symbolized as ACP, was first isolated in pure form from E. coli and
has since been studied from many other sources. The E. coli ACP is a relatively
small (mol wt 10,000), heat-stable protein containing 77 amino acid residues,
whose sequence has been established, and a covalently attached prosthetic group.
The single sulfhydryl group of
ACP, to which the acyl intermediates are esterified, is contributed by its
prosthetic group, a molecule of 4'-phosphopantetheine,
which is covalently linked to the hydroxyl group of serine residue 36 of the
protein. The 4'-phosphopantetheme moiety is identical with that of coenzyme A,
from which it is derived. The function of ACP in fatty acid synthesis is
analogous to that of CoA in fatty acid oxidation: it serves as an anchor to
which the acyl intermediates are esterified.
The priming reaction
To prime the fatty-acid
synthetase system, acetyl-CoA first reacts with the sulfhydryl group of ACP by
the action of one of the six enzymes of the synthetase system, ACP-acyltransferase, which catalyzes the
reaction:
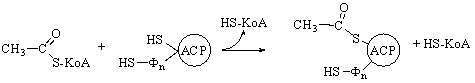
The malonyl transfer step
In
the next reaction, catalyzed by ACP
malonyltransferase, malonyl-S-CoA formed in the acetyl-CoA carboxylase reaction
reacts with the —SH group of the 4'-phosphopantetheine arm of ACP, with loss of
free CoA, to form malonyl-S-ACP:
Malonyl—S—CoA
+ ACP—SH Û malonyl—S—ACP + CoA—SH
As a result of this step and of the
preceding priming reaction, a malonyl group is now esterified to ACP and an
acetyl group is esterified to an —SH group on the ACP molecule.
The condensation reaction
In the next reaction of the
sequence, catalyzed by b-ketoacyl-ACP synthase, the acetyl group esterified to the cysteine residue is transferred to
carbon atom 2 of the malonyl group on ACP, with release of the free carboxyl
group of the malonyl residue as CO2:
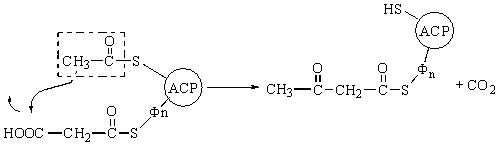
Study of the reaction equilibrium has revealed
the probable basis for the biological selection of malonyl-CoA as the
precursor of two-carbon residues for fatty acid synthesis. If acetoacetyl-CoA
were to be formed from two molecules of acetyl-CoA by the action of acetyl-CoA
acetyltransferase,
Acetyl—S—CoA + acetyl—S—CoA Û
acetoacetyl—S—CoA + CoA—SH
the
reaction would be endergonic, with its equilibrium lying to the left.
The first reduction reaction
The acetoacetyl-S-ACP
now undergoes reduction by NADPH to form b-hydroxybutyryl-S-ACP. This reaction is catalyzed by b-ketoacyl-ACP
reductase:
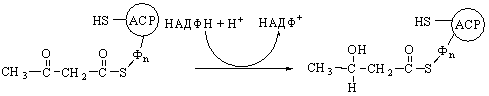
The dehydration step
b-Hydroxybutyryl-S-ACP is next dehydrated to the corresponding unsaturated acyl-S-ACP, namely, crotonyl-S-ACP, by b-hydroxyacyl—ACP-dehydratase:
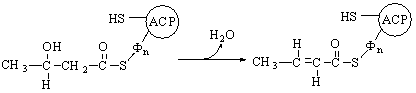
The second reduction step
Crotonyl-S-ACP is now
reduced to butyryl-S-ACP by enoil-ACP reductase (NADPH); the electron donor is NADPH in animal tissues:
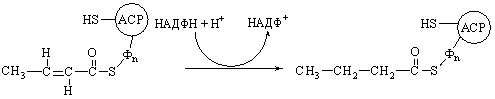
Crotonyl-S-ACP Butyryl-S-ACP
This reaction also differs
from the corresponding reaction of fatty acid oxidation in mitochondria in that
a pyridine nucleotide rather than a flavoprotein is involved. Since the
NADPH-NADP+ couple has a more negative standard potential than the
fatty acid oxidizing flavoprotein, NADPH favors reductive formation of the saturated
fatty acid.
The formation of butyryl-ACP completes the first of seven
cycles en route to palmitoyl-S-ACP.
To start the next cycle the butyryl group is transferred from —SH group of
phosphopantetheine to the —SH group of cysteine, thus allowing —SH group of ACP
phosphopantetheine to accept a malonyl group from another molecule of malonyl-CoA.
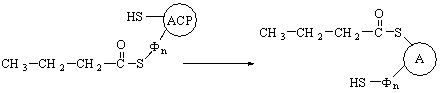
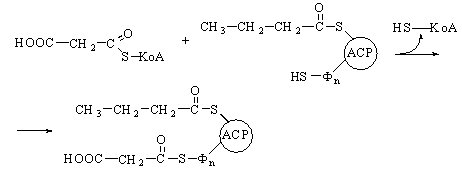
Then the cycle repeats, the next step
being the condensation of malonyl-S-ACP with butyryl-S-ACP to yield b-ketohexanoyl-S-ACP and CO2.
After seven complete cycles, palmitoyl-ACP is the end product. The
palmitoyl group may be removed to yield free palmitic acid by the action of a thioesterase, or it may be transferred from
ACP to CoA, or it may be incorporated directly into phosphatidic acid in the
pathway to phospholipids and triacylglycerols.
It
is remarkable that in most organisms the fatty-acid synthetase system stops
with the production of palmitic acid and does not yield stearic acid, which has
only two more carbon atoms than palmitic acid and thus does not differ greatly
in physical properties.
Saturated fatty acids having an odd
number of carbon atoms, which are found in many marine organisms, are also made
by the fatty-acid synthetase complex. In this case the synthesis is primed by a
starter molecule of propionyl-S-ACP (instead of acetyl-S-ACP), to which are
added successive two-carbon units via condensations with malonyl-S-ACP.
We
can now write the overall equation for palmitic acid biosynthesis starting from
acetyl-S-CoA:
8 Acetyl—S—CoA + 14NADPH
+ 14H+ + 7ATP + H2O ®
palmitic acid + 8CoA + 14NADP+ + 7ADP + 7P.
The 14 molecules of NADPH
required for the reductive steps in the synthesis of palmitic acid arise largely
from the NADP-dependent oxidation of
glucose 6-phosphate via the phosphogluconate
pathway. Liver, mammary gland, and adipose tissue of vertebrates, which have a
rather high rate of fatty acid biosynthesis, also have a very active
6-phosphogluconate pathway.
The
enzymatic steps leading to the biosynthesis of palmitic acid differ from those
involved in oxidation of palmitic acid in the following respects:
1. Their intracellular
location.
2. The type of acyl-group
carrier.
3. The form in which the two-carbon
units are added or removed.
4. The pyridine nucleotide
specificity of the b-ketoacyl-b-hydroxyacyl reaction.
5. The stereoisomeric configuration
of the b-hydroxyacyl intermediate.
6. The electron donor-acceptor
system for the crotonyl-butyryl step.
These differences illustrate how two
opposing metabolic processes may proceed independently of each other in the
cell.
Elongation of saturated fatty
acids in mitochondria and microsomes
Palmitic acid, the normal end product of the
fatty-acid synthetase system, is the precursor of the other long-chain saturated
and unsaturated fatty acids in most organisms. Elongation of palmitic acid to
longer-chain saturated fatty acids, of which stearic acid is most abundant, occurs by the action of two
different types of enzyme systems, one in the mitochondria and the other in the
endoplasmic reticulum.
In mitochondria palmitic and other
saturated fatty acids are lengthened by successive additions to the
carboxyl-terminal end of acetyl units in the form of acetyl-CoA; malonyl-ACP
cannot replace acetyl-CoA. The mitochondrial elongation pathway occurs by
reactions similar to those in fatty acid oxidation. Condensation of
palmityl-CoA with acetyl-CoA yields b-ketostearyl-CoA, which is reduced by NADPH to b-hydroxystearyl-CoA. The latter is dehydrated to the
unsaturated stearyl-CoA, which is then reduced to yield stearyl-CoA at the
expense of NADPH. This system will also elongate unsaturated fatty acids.
Microsome
preparations can elongate both saturated and unsaturated fatty acyl-CoA esters,
but in this case malonyl-CoA rather than acetyl-CoA serves as source of the
acetyl groups. The reaction sequence is identical to that in the fatty-acid
synthetase system except that the microsomal system employs CoA and not ACP as acyl
carrier.
Formation
of monoenoic acids
Palmitic and
stearic acids serve as precursors of the two common monoenoic (monounsaturated) fatty acids of animal tissues, namely, poimitoleic and oleic acids, both of which possess a cis double bond in the D9 position. Although most organisms can form
palmitoleic and oleic acids, the pathway and enzymes employed differ between
aerobic and anaerobic organisms. In
vertebrates (and most other aerobic organisms) the D9 double
bond is introduced by a specific monooxygenase system; it is located in the
endoplasmic reticulum of liver and adipose tissue. One molecule of molecular
oxygen (O2) is used as the acceptor for two pairs of electrons, one
pair derived from the palmitoyl-CoA or stearyl-CoA substrate and the other from
NADPH, which is a required coreductant in the reaction. The transfer of electrons
in this complex reaction involves a microsomal electron-transport chain which
carries electrons from NADPH (or NADH) to microsomal cytochrome b5 via cytochrome
b5 reductase, a
flavoprotein. A terminal cyanide-sensitive factor (CSF), a protein, is required
to activate the acyl-CoA and the oxygen.
The overall reaction for palmitoyl-CoA is:
Palmitoyl—CoA + NADPH + H+ + O2 ® palmitoleyl—CoA + NADP+ + 2H2O.
Formation of
polyenoic acids
Bacteria do not contain
polyenoic acids; however, these acids are abundant both in higher plants and in
animals. Mammals contain four distinct families of polyenoic acids, which
differ in the number of carbon atoms between the terminal methyl group and the
nearest double bond. These families are named from their precursor fatty
acids, namely, palmitoleic, oleic,
linoleic, and linolenic acids. All polyenoic acids found in mammals are
formed from these four precursors by further elongation and/or desaturation
reactions. Two of these precursor fatty acids, linoleic and linolenic acids,
cannot be synthesized by mammals and must be obtained from plant sources; they
are therefore called essential fatty
acids.
The elongation of chains of polyenoic
acids occurs at the carboxyl end by the mitochondrial or microsomal systems
described above. The desaturation steps occur by the action of the cytochrome b5-oxygenase
system with NADPH as coreductant of oxygen, like the steps in the formation of
palmitoleic and oleic acids, also described above.
Arachidonic acid is the most abundant polyenoic acid. When young rats are placed on
diets deficient in essential fatty acids, they grow slowly and develop a scaly
dermatitis and thickening of the skin. This condition can be relieved by
administration not only of linoleic or linolenic acid but also of arachidonic
acid. The essential fatty acids and some of their derivatives serve as precursors of the prostaglandins.
In
plants linoleic and linolenic acids are synthesized from oleic acid via aerobic
desaturation reactions catalyzed by specific oxygenase systems requiring NADPH
as coreductant.
The
double bonds of naturally occurring fatty acids do not in general undergo
hydrogenation to yield more completely saturated fatty acids; only a few
organisms appear to carry out this process. Unsaturated fatty acids, however,
are completely oxidized by the fatty acid oxidation system.
In
most organisms the conversion of saturated to unsaturated fatty acids is
promoted by low environmental temperatures. This is an adaptation to maintain
the melting point of the total cell lipids below the ambient temperature;
unsatu-rated fatty acids have lower melting points than saturated. In some
organisms the enzymes involved in fatty acid desaturation increase in
concentration in response to low temperatures; in others the unsaturated fatty
acids are inserted into lipids at increased rates.
Biosynthesis of triacylglycerols
The triacylglycerols, which function as depot, or storage, lipids, are
actively synthesized in the cells of vertebrates, particularly liver and fat
cells, as well as those of higher plants. Bacteria in general contain
relatively small amounts of triacyglycerols.
In higher animals and plants two
major precursors are required for the synthesis of triacylglycerols: L-glycerol 3-phosphate and fatty acyl-CoA.. L-Glycerol 3-phosphate is derived from two different sources. Its
normal precursor is dihydroxyacetone
phosphate, the product of the aldolase
reaction of glycolysis. Dihydroxyacetone phosphate is reduced to L-glycerol
3-phosphate by the NAD-linked glycerol-
3-phosphate dehydrogenase of the cytosol:
Dihydroxyacetone phosphate + NADH + H+
® L-glycerol 3-phosphate + NAD+
It may also be formed from free glycerol
arising from degradation of triacylglycerols, through the action of glycerol kinase:
ATP + glycerol ® L-glycerol 3-phosphate + ADP
The first stage in
triacyglycerol formation is the acylation of the free hydroxyl groups of
glycerol phosphate by two molecules of fatty acyl-CoA to yield first a lysophosphotidic acid and then a phosphatidic acid:
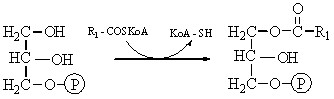
Glycerol
phosphate Lysophosphotidic
acid 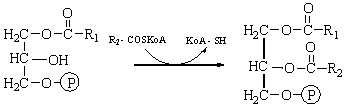
Lysophosphotidic acid
Phosphatidic acid
Free glycerol is not
acylated. These reactions occur preferentially with 16- and 18-carbon saturated
and unsaturated acyl-CoA.
Phosphatidic
acids occur only in trace amounts in cells, but they are important
intermediates in the biosynthesis of triacylglycerols and phosphoglycerides.
In the pathway to
triacylglycerols, phosphatidic acid undergoes hydrolysis by phosphatidate phosphatase to form a diacylglycerol:
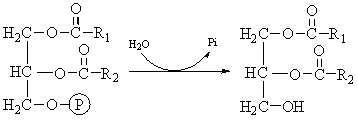
Phosphatidic acid Diacylglycerol
The diacylglycerol then reacts with a
third molecule of a fatty acyl-CoA to yield a triacylglycerol by the action of diacylglycerol acyltransferase:

Diacylglycerol Triacylglycerol
In the intestinal mucosa of higher
animals, which actively synthesizes triacylglycerols during absorption of fatty
acids from the intestine, another type of acylation reaction comes into play.
Monoacylglycerols formed during intestinal digestion may be acylated directly
by acylglycerol palmitoyltrans-ferase
and thus phosphatidic acid is not an intermediate:
Monoacylglycerol + palmitoyl-CoA ® diacylglycerol + CoA
In
storage fats of animal and plant tissues the triacylglycerols are usually
mixed, i.e., contain two or more different fatty acids.