Management of Patients with Hypertensive Crises
Affecting one
quarter of the adult population (60 million in the United States and 1 billion
people worldwide), arterial hypertension is the leading cause of death in the
world and the most common cause for an outpatient visit to a physician; it is
the most easily recognized treatable risk factor for stroke, myocardial
infarction, heart failure, peripheral vascular disease, aortic dissection, atrial fibrillation, and end-stage kidney disease.
Despite this knowledge and unequivocal scientific proof that treatment of
hypertension can prevent many of its life-altering complications, hypertension
remains untreated or undertreated in the majority of affected individuals in
all countries, including those with the most advanced systems of medical care.
Inadequate treatment of hypertension is a major factor contributing to some of
the adverse secular trends since the early 1990s, including an increased incidence
of stroke, heart failure, and kidney failure plus a leveling off
of the decline in coronary heart disease mortality.
Across populations, the risks of
heart disease and stroke increase continuously and logarithmically with
increasing levels of systolic and diastolic blood pressure at or above 115/75
mm Hg . Thus, the dichotomous separation of “normal”
from “high” blood pressure is artificial, and the definition of arterial
hypertension (i.e., high blood pressure) has been a moving target. On the basis of results of randomized clinical drug trials, hypertension
currently is defined as a usual blood pressure of 140/90 mm Hg or higher, the
value above which the benefits of treatment appear to outweigh the risks. Prehypertension is a new designation for mildly
elevated blood pressures between 120/80 and 139/89 mm Hg, a level at which
progression to hypertension is twice as likely as with a blood pressure below
120/80 mm Hg, and cardiovascular risk retains its continuous log-linear
function compared with lower blood pressures. The cardiovascular mortality rate
is only half as great at 120/80 mm Hg as at 140/90 mm Hg, but it is unknown
whether the benefits of treating prehypertension
outweigh the risks.
Arterial hypertension, defined as a
systolic blood pressure (SBP) in excess of 140 mm Hg and/or diastolic
blood pressure (DBP) in excess of 90
mm Hg, has long been identified
as an independent risk factor for cardiovascular disease. Traditionally,
emphasis has been placed on elevated DBP as a risk
factor for the development of target organ damage. However, as early as 1971,
the Framingham study showed that, although DBP was a major determinant of
cardiovascular risk in men under 45 years of age, SBP was the stronger risk
factor in older men and in women of all ages. Since then,
several observational studies have suggested that the pulse pressure (PP) may
be a better predictor of cardiovascular complications than SBP or mean arterial
pressure.
Data from the National Health and
Nutrition Examination Survey has demonstrated that if a blood pressure (BP) of
140/90 mm Hg is considered to be normal, only 27% of
hypertensive patients are adequately controlled in the United States.
Recommendations from the Joint National Committee on the Prevention, Detection,
Evaluation and treatment of High Blood Pressure (JNC-VI report) now regard a BP
of 140/90 mm Hg as high normal and 130/85 mm Hg as normal. For diabetic patients, therefore, it is recommended that BP be
reduced below 130/85 mm Hg and for those with renal impairment, evidenced by proteinuria,
pressures should be reduced below 125/75 mm Hg. In patients with underlying
coronary artery disease, the BP should be reduced
below 120/80 mm Hg.
The beating heart generates pressure
and flow waves which propagate throughout the arterial
system. The shape of the pressure and flow waves is altered
by their continuous interaction with the non-uniform arterial system. The
pressure and flow waves can be studied in terms of a
forward component, running from the heart itself, and a backward component
carrying information on the peripheral arterial system.
In the presence of arteriosclerosis
and aortic stiffening (consequences of arterial hypertension), the pulse wave
velocity is increased, causing the pulse waves to reflect more quickly off the
arteriolar vessels and return to the large vessels during systole. This
amplifies SBP. In the presence of normal vascular compliance, the reflected
waves return during diastole and augment DBP. Consequently, arteriosclerosis
tends simultaneously to increase SBP and decrease DBP, resulting in a widened
pulse pressure.
A widened pulse pressure increases
cardiovascular morbidity because elevated SBP is associated with greater left
ventricular workload and myocardial oxygen demand, whereas a decreased DBP may
decrease coronary perfusion, resulting in decreased myocardial oxygen supply
and a greater risk for myocardial ischemia and injury.
Hypertension (systolic pressure  140 mm Hg or
diastolic pressure
140 mm Hg or
diastolic pressure  90 mm Hg) is present in one in four adults in the United
States.1
The prevalence is higher among blacks
and older persons, especially older women. Table 1 shows the classification of
blood pressure according to the Joint National Committee on
Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.2 Hypertension is a risk
factor for stroke, myocardial infarction, renal failure, congestive
heart failure, progressive atherosclerosis, and dementia.3
Systolic pressure
is a stronger predictor of
cardiovascular events than is diastolic pressure,4 and isolated systolic hypertension, which
is common among older persons, is particularly hazardous.5
There is a
continuous, graded relation between blood pressure and the risk of cardiovascular disease;
the level and duration of
hypertension and the
presence or absence of
coexisting cardiovascular risk factors determine the outcome.6 Treatment of hypertension reduces the risk of stroke, coronary artery disease, and congestive
heart failure, as well as overall cardiovascular morbidity and mortality
from cardiovascular causes. However, only 54 percent of patients with hypertension receive treatment and only 28 percent
have adequately controlled blood pressure.1
90 mm Hg) is present in one in four adults in the United
States.1
The prevalence is higher among blacks
and older persons, especially older women. Table 1 shows the classification of
blood pressure according to the Joint National Committee on
Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.2 Hypertension is a risk
factor for stroke, myocardial infarction, renal failure, congestive
heart failure, progressive atherosclerosis, and dementia.3
Systolic pressure
is a stronger predictor of
cardiovascular events than is diastolic pressure,4 and isolated systolic hypertension, which
is common among older persons, is particularly hazardous.5
There is a
continuous, graded relation between blood pressure and the risk of cardiovascular disease;
the level and duration of
hypertension and the
presence or absence of
coexisting cardiovascular risk factors determine the outcome.6 Treatment of hypertension reduces the risk of stroke, coronary artery disease, and congestive
heart failure, as well as overall cardiovascular morbidity and mortality
from cardiovascular causes. However, only 54 percent of patients with hypertension receive treatment and only 28 percent
have adequately controlled blood pressure.1

Strategies and Evidence
Evaluation
Accurate measurement of
blood pressure7 and verification of elevated pressure on multiple occasions
over time are important. Ambulatory or home blood-pressure
monitoring8 can identify "white-coat hypertension" (blood pressure
that is elevated when measured during an office visit but that is otherwise
normal) and prevent unnecessary treatment. White-coat hypertension, present in 20 percent
of patients with
elevated blood pressure, is associated with a lower cardiovascular
risk than is sustained hypertension,
but it may be a precursor of
sustained hypertension
and therefore warrants monitoring.
In addition to the history taking and physical examination, several
tests are routinely indicated in patients with hypertension: urinalysis, complete blood
count, blood chemical tests (measurements of potassium, sodium, creatinine, fasting glucose, total cholesterol, and
high-density lipoprotein), and 12-lead electrocardiography. The
evaluation should identify signs of
cardiovascular, cerebrovascular, or
peripheral vascular disease and other cardiovascular risk factors
that are frequently present in patients with hypertension. Severe or resistant hypertension or clinical or
laboratory findings suggesting the presence of renal disease, adrenal hypertension (due to
abnormal mineralocorticoid secretion or pheochromocytoma), or renovascular
hypertension should be
further investigated. Essential, or primary, hypertension, the focus of this article, is the
diagnosis in over 90 percent of
cases.
Video:
hypertensive heart
Treatment
The primary goal of
the treatment of hypertension is to prevent cardiovascular
disease and death. Coexisting cardiovascular risk factors increase
the risks associated with hypertension
and warrant more aggressive treatment.
The five-year risk of
a major cardiovascular event in a 50-year-old man with a blood pressure
of 160/110 mm Hg is
2.5 to 5.0 percent; the risk doubles if the man has a high
cholesterol level and triples if he is also a smoker.9
The benefits of
lowering blood pressure, first demonstrated after short-term treatment of malignant hypertension,10 have subsequently been demonstrated in all
stages of hypertension. Trials
involving patients with stage 1 or 2 hypertension
showed that lowering systolic pressure by 10 to 12 mm Hg and diastolic pressure
by 5 to 6 mm
Hg reduces the risk of
stroke by 40 percent, the risk of coronary disease by 16 percent, and the risk of death from any
cardiovascular cause by 20 percent.11,12 The higher the blood pressure and the
number of risk
factors, the greater the reduction in absolute risk (and the smaller
the number needed to treat).
Determination of
the need for drug therapy is based on a combined assessment of the blood-pressure level and the
absolute risk of
cardiovascular disease (Figure 1). Patients with stage 1 hypertension can
be treated with lifestyle modifications alone for up to one
year, if they have no other risk factors, or for up to six months,
if they have other risk factors. Drug treatment
should be provided if blood pressure remains
elevated after a trial of
lifestyle modifications alone. Lifestyle modifications and
antihypertensive therapy are indicated for patients
with cardiovascular or other target-organ disease (renal, cardiac,
cerebrovascular, or retinal disease) and for
those with stage 2 or 3 hypertension.
Patients with diabetes are at high risk, and drug therapy is indicated in such patients even if blood pressure
is at the high end of
the normal range.

Figure 1. Treatment of Hypertension According to the Level of
Blood Pressure and Cardiovascular Risk.
Two or more blood-pressure
readings separated by two minutes should be averaged.
If the pressure is at the high end of the normal range, it should
be rechecked yearly. Stage 1 hypertension should be confirmed
within two months. Patients with stage 2 hypertension should
be evaluated and referred for care within one month; those with stage 3
hypertension should be evaluated immediately or within one week. If systolic
and diastolic values are in different categories, the recommendations for the
higher reading should be followed.
Laboratory tests include a
complete blood count; measurements of potassium, sodium, creatinine,
fasting glucose, total cholesterol, and high-density lipoprotein cholesterol;
and urinalysis. ECG denotes electrocardiography. Cardiovascular or other
target-organ disease denotes left ventricular hypertrophy, angina or prior
myocardial infarction, prior coronary revascularization, heart failure, stroke
or transient ischemic attack, nephropathy, peripheral arterial disease, and
retinopathy.
For patients with multiple
risk factors, clinicians should consider drugs as initial therapy along with
lifestyle modifications. Clinically important risk factors include smoking, dyslipidemia, diabetes mellitus, an
age of more than 60 years, male sex, postmenopausal status in women, and a
family history of cardiovascular disease for women under the age of 65 years
and men under the age of 55 years. Adapted from the Joint National Committee on
Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.2
Lifestyle Modifications
Table 2 lists lifestyle modifications recommended for all patients with
hypertension. The
Dietary Approaches to Stop Hypertension
(DASH) study showed that eight weeks of a diet of
fruits, vegetables, low-fat dairy products, whole grains, poultry,
fish, and nuts, with limited fats, red meat, and sweets, reduced
systolic pressure by 11.4
mm Hg and diastolic pressure by 5.5 mm Hg.13 With sodium intake at a level below 100 mmol per day, systolic pressure was 3 mm Hg lower and diastolic
pressure was 1.6 mm
Hg lower than with the DASH diet and a higher level of sodium intake.14

Restriction of sodium intake to 2 g per day lowers systolic
pressure, on average, by 3.7 to 4.8 mm Hg and lowers diastolic pressure,
on average, by 0.9 to 2.5 mm
Hg,15,16 although the reductions vary from person
to person beyond these ranges. Salt sensitivity is common in elderly
patients with hypertension.
Despite concern that salt restriction for all patients with hypertension might have
adverse consequences,17 moderate sodium restriction appears to be
generally safe and effective18 and is particularly effective in elderly
persons.19
Whether lifestyle
modifications can be sustained is a concern. Four
years after enrollment in the Treatment of Mild Hypertension Study,
patients with stage 1 hypertension
had gained back half the weight lost after one year of intervention and were less successful
at maintaining a low sodium intake and an increased level of physical activity than
they had been at one year.20 Nevertheless, lifestyle modifications alone controlled blood pressure
at four years in 59 percent of
the patients.
Most clinical trials
of lifestyle
modifications have been underpowered or of insufficient duration to evaluate
the effect of these
interventions on major cardiovascular outcomes. However, lifestyle modifications
should be encouraged, since they are safe and inexpensive and, when
combined with drug therapy, may result in better blood pressure
control and an improved quality of
life.21
Treatment Goal for Blood
Pressure
The risk of cardiovascular disease remains
higher in treated patients with hypertension than in persons with normal blood pressure,
suggesting that treatment
targets have not been low enough. Greater reductions in blood
pressure have been shown to be safe and beneficial.22,23 In the Hypertension
Optimal Treatment
trial, the risk of
major cardiovascular events was lowest among patients whose blood
pressure had been reduced to 138.5/82.6 mm Hg. An additional
reduction did not further
reduce the risk of
events in nondiabetic patients, but it was not
harmful. Among diabetic patients, the lowest rates of major cardiovascular
events and death from cardiovascular causes were achieved
with the lowest blood pressure. In patients over the age of 65 years, morbidity and mortality
from cardiovascular disease are reduced when systolic pressure is
lowered to a level below 160 mm Hg.24 Whether levels below 140 mm Hg provide additional
protection is unclear.
Choice
of Antihypertensive
Drugs
Most
antihypertensive drugs reduce blood pressure by 10 to 15 percent. Monotherapy is effective in about 50 percent of unselected patients,
and those with stage 2 or 3 hypertension
often need more
than one drug.25 There have been few
comparative trials of
antihypertensive agents that have had sufficient power to demonstrate
an advantage of one
drug over another, and there is individual variation in
responsiveness to drugs. Thus, the choice of therapy is
based on a combined assessment of
several characteristics of
the patient: coexisting conditions, age, race or ethnic group, and
the response to previously used drugs, including the presence or
absence of adverse
reactions.
A critical issue is
whether a drug reduces cardiovascular morbidity and mortality. As
compared with placebo, diuretics and beta-blockers reduce the risk of stroke, coronary heart disease,
and overall mortality from cardiovascular disease in unselected
patients with hypertension
who do not have preexisting coronary disease, diabetes,
or proteinuria.11,12 A meta-analysis of trials involving more than 26,000
patients showed that, as compared with placebo, angiotensin-converting–enzyme
(ACE) inhibitors reduce the risk of stroke, coronary heart disease, major
cardiovascular events, death from cardiovascular causes, and death
from any cause,26 although the results were heavily dependent on a
trial in which all the participants had preexisting
cardiovascular disease or diabetes and some did not have hypertension.27
Calcium-channel antagonists, as
compared with placebo, reduce the risk of
stroke, major cardiovascular events, and death from
cardiovascular causes; however, these drugs do not significantly
reduce the risk of
coronary heart disease, heart failure, or death from any cause.26
The question of whether antihypertensive agents
differ in their ability to prevent adverse outcomes has been
difficult to answer.28
Some data suggest potentially important
differences. For example, ACE inhibitors were more effective than
calcium-channel antagonists in preventing coronary heart disease in
one trial,29 but not in another, larger study.30 A meta-analysis of clinical trials suggests that ACE
inhibitors are more effective than calcium-channel antagonists in
reducing the risk of
heart failure but not in reducing the risk of stroke, death from cardiovascular
disease, or death from any cause.26 Losartan, an angiotensin-receptor antagonist, has recently
been shown to be more effective than atenolol
in reducing the risk of stroke.31 Another meta-analysis suggests that
calcium-channel antagonists may prevent stroke to a greater extent
than diuretics or beta-blockers but have not been shown to provide
similar protection against coronary heart disease.32 The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack
Trial, the largest randomized trial comparing several
antihypertensive agents as initial
therapy, demonstrated that in patients older than 55 years (35
percent of
whom were black and 19 percent of
whom were Hispanic), diuretic-based therapy was as effective as treatment with calcium-channel
antagonists or ACE inhibitors in preventing major coronary events.33
Diuretic-based therapy was slightly
more effective than treatment
with calcium-channel antagonists in preventing heart failure and
was more effective than treatment
with ACE inhibitors in preventing stroke and heart failure. A
smaller study of elderly
white men and women with hypertension,
showed that ACE-inhibitor–based therapy was slightly more effective
than diuretic-based therapy in preventing myocardial infarction (only
in men) but not stroke.34
On the basis of the available data, diuretics or beta-blockers remain
appropriate for the initial
treatment of uncomplicated hypertension, despite the concern
that these agents may be associated with adverse metabolic effects
(e.g., hyperuricemia and impaired glucose
tolerance). Alternative drugs are preferable for patients with
certain coexisting medical conditions (Table 3). In particular, ACE inhibitors and angiotensin-receptor antagonists are appropriate initial therapy in patients with
diabetes mellitus, renal disease, or congestive heart failure35,36 (though beta-blockers and diuretics are
also useful in patients with heart failure); ACE inhibitors can also
be used in patients with prior myocardial infarction or coronary
artery disease. Short-acting calcium-channel antagonists cause a
rapid, acute drop in blood pressure, which may precipitate coronary
ischemia, and long-acting calcium-channel antagonists are therefore
preferred when this class of agent is chosen.37 Alpha-blockers relieve symptoms associated with prostatic hypertrophy.
Since they are not as effective as other agents in reducing the
risk of cardiovascular disease, they should be used as second-
or third-line therapy.33

Other
Considerations in the Choice of Therapy
Age and race have been shown to be determinants of the response to
specific antihypertensive medications. The Department of Veterans
Affairs Cooperative Study reported that younger whites had a good
response to ACE inhibitors and beta-blockers, whereas older blacks
had a better response to diuretics or calcium-channel antagonists.25
Hypertension is more
severe and target-organ damage, particularly end-stage renal
disease, more prevalent among blacks. Salt sensitivity is common,
and sodium restriction should be encouraged. Although the
magnitude of the blood-pressure response to monotherapy
with a diuretic or a calcium-channel antagonist may be greater than
the response to monotherapy with another agent,
significant reductions occur with ACE inhibitors, angiotensin-receptor antagonists, and
beta-blockers when an adequate dose is given.38
Side effects differ according
to the class of antihypertensive drug (Table 3). Although
adverse effects are reported by 10 to 20 percent of patients taking
such drugs, the quality of life improves when hypertension is
treated.21
The Treatment of Mild Hypertension Study and the Department of
Veterans Affairs Cooperative Study both demonstrated that among the
five main classes of antihypertensive drugs (diuretics,
beta-blockers, calcium-channel antagonists, ACE inhibitors, and
alpha-blockers), no one drug is more acceptable than the others,
except that sexual dysfunction is more common among men treated with
the diuretic chlorthalidone.21,25
Use of lower-cost, generic drugs that require less frequent doses
can improve compliance.
Combination
Therapy
The use of lower doses of two
or more drugs with complementary mechanisms may lower blood pressure
with fewer adverse effects than the use of higher doses of a single
agent. Most combination therapies include small doses
of a diuretic, which potentiate the effects of other drugs (ACE
inhibitors, angiotensin-receptor antagonists,
or beta-blockers). Combination therapy may improve compliance and achieve
the target blood pressure more rapidly.
Guidelines
National and international
groups have issued guidelines for the treatment of hypertension. The
main differences among these guidelines are the criteria for
initiating drug therapy in low-risk patients with stage 1
hypertension. The Joint National Committee on
Prevention, Detection, Evaluation, and Treatment of High Blood
Pressure2
and the World Health Organization–International Society of
Hypertension41
recommend stratification of patients into risk categories on the
basis of age, sex, smoking status, presence or absence of diabetes,
cholesterol level, presence or absence of preexisting
cardiovascular disease, and presence or absence of target-organ
damage (Figure 1). Drug treatment
is recommended for stage 1 or higher
hypertension if blood pressure does not decrease after a certain
period of lifestyle-modification counseling
(6 to 12 months, according to the Joint National Committee
guidelines). The British Hypertension Society and New Zealand
guidelines recommend the use of tables that quantify a person's 5-
or 10-year risk of a cardiovascular event; drugs are recommended
only if the 5-year risk is at least 10 percent.42,43
When drugs are indicated, the guidelines recommend
those that have been shown to improve cardiovascular outcomes, with
coexisting conditions and demographic characteristics taken into
account.
Areas of Uncertainty
Although moderate sodium
restriction lowers blood pressure, the small effects, variability in
response, and lack of a proven cardiovascular benefit have led to
uncertainty about whether it should be broadly
recommended. There is also uncertainty about whether specific
properties of certain drugs result in differential effects on
morbidity and mortality that are independent of the reduction in
blood pressure.
The use of drugs in patients
with a low absolute risk of cardiovascular disease is controversial.
The rationale for withholding drugs from such patients is that some
trials have shown that mortality among low-risk patients treated
with drugs is similar to that in control groups.44
However, given that even high-normal blood pressures (130 to 139/85
to 89 mm
Hg) are associated with an increased risk of cardiovascular disease,45
there is concern about withholding drugs from "low-risk"
patients. Also, the feasibility of basing
treatment decisions on the use of tables for calculating the
absolute risk of cardiovascular disease has not been assessed.
The appropriate strategy for
choosing the initial antihypertensive therapy is still unresolved.
Some have proposed that the choice of treatment should be based on renin levels,46
but this approach is not widely used. Whether combination therapy as
the initial treatment leads to better control of blood pressure and
a lower risk of cardiovascular disease than monotherapy
is also unresolved. Finally, optimal blood-pressure targets remain
to be determined, particularly for elderly patients.
Conclusions and
Recommendations
Hypertension affects 25
percent of adults in the United
States and is
adequately treated in less than 30 percent of them. Appropriate therapy
can reduce blood pressure and cardiovascular morbidity and
mortality.
Persons who
have stage 1 hypertension and are at low risk for cardiovascular
disease can
be treated with lifestyle
modifications for up to one year. Patients
who have stage 1 hypertension and other cardiovascular risk factors
or a higher stage of hypertension should be treated with drugs to reduce blood pressure to a level below 140/90 mm
Hg, or to reduce pressure to 130/80 mm Hg or less if the patient has
diabetes, renal disease, or both.
Diuretics and
beta-blockers are appropriate as first-line therapy for patients without
coexisting conditions. ACE
inhibitors or angiotensin-receptor
antagonists are recommended for patients with type 2 diabetes,
kidney disease, or both and are also useful in
patients with heart failure. Beta-blockers and ACE inhibitors are recommended in patients with prior myocardial
infarction, and calcium-channel antagonists benefit elderly patients
at risk for stroke. If blood pressure is not
controlled with an optimal dose of a single drug, a second
agent with a complementary mechanism of action should be added. Combination therapy provides more rapid control of blood
pressure than does monotherapy and is
therefore an initial treatment option for patients with stage 2 or 3
hypertension.
Resistant or Difficult-to-Control Hypertension
Case vignette. A 70-year-old woman
with a long-standing history
of hypertension comes
for follow-up. Her
medications include atenolol (100 mg daily),
hydrochlorothiazide
(12.5 mg daily), lisinopril (40 mg daily),
and ibuprofen (400 mg twice daily for
osteoarthritis). She does not smoke or drink alcohol. Her body-mass index (the weight
in kilograms divided by the square of the height in meters) is 32.
Her systolic and diastolic blood pressures (measured
three times while she was seated) range from 164
to 170 mm Hg and 92 to 96 mm Hg, respectively, and
the pulse rate is 72 per minute. Examination of her ocular fundi reveals arteriolar narrowing. The results
of cardiovascular examination are normal.
There are no abdominal bruits. The serum potassium level is 3.8 meq per liter, and the
serum creatinine level is 1.2 mg per deciliter (106 µmol per liter);
there is no microalbuminuria. How should
this patient be further evaluated and treated?
The Clinical Problem
Resistant, or refractory, hypertension is defined by a blood
pressure of at least 140/90 mm Hg or
at least 130/80 mm Hg in patients with diabetes or renal disease (i.e., with a creatinine level of more than 1.5 mg per deciliter [133 µmol per liter]
or urinary protein
excretion of more than
300 mg over a 24-hour period), despite adherence to treatment with full doses
of at least three antihypertensive medications, including a
diuretic. Patients who have recently
received a diagnosis of hypertension
or who have not yet
received treatment should not be considered
to have resistant hypertension, regardless of
their blood-pressure level.
Data on the
prevalence of resistant
hypertension are
scant. In large clinical trials of hypertension in which protocols required drug
titration until the blood pressure was below a predefined target,
the diastolic blood
pressure was below 90 mm
Hg in approximately 90 percent of patients, but the systolic blood pressure was
below 140 mm
Hg in only 60 percent of patients. However, patients who had no
predefined response to
treatment did not meet all of the criteria for resistant hypertension
as cited above. In one specialty hypertension
clinic, only 59 percent of patients whose hypertension was considered to be
resistant
had blood pressures below 140/90 mm Hg despite careful drug
titration. These observations suggest that blood-pressure goals may
be difficult to achieve in as many as 40 percent
of patients. Resistant
or difficult-to-control systolic
hypertension is
more common in
patients over the age of 60 years than in younger patients.
Patients whose hypertension is uncontrolled are more likely to have target-organ damage and a higher long-term
cardiovascular risk than are patients whose blood pressure is controlled. Heart
failure, stroke, myocardial infarction, and renal failure are related to
the degree of the elevation in blood pressure. Other risk factors, such as diabetes and dyslipidemia, further increase the
cardiovascular risk in these patients.
Difficult-to-control hypertension is
defined here as persistently elevated blood pressure despite treatment
with two or three
drugs but not meeting the above-mentioned strict criteria for resistant hypertension.
Difficult-to-control hypertension is far more common than resistant
hypertension.
Strategies and Evidence
Formal studies of the management of resistant or difficult-to-control hypertension are few, and strategies
are based largely on observational data
from specialty clinics. These series and clinical experiences suggest
that a careful evaluation of a patient's adherence to and adequacy of
therapy and lifestyle factors
often reveals modifiable contributors to
refractory blood
pressure; secondary causes (including exogenous substances) must also be considered. A suboptimal medical
regimen has been shown to be the primary cause of resistant hypertension in a majority of patients in these
studies. Figure 1 outlines a suggested approach to
evaluation.


Diagnosis
Blood pressure should be measured after a patient has been seated quietly
for five minutes, with his or her arm supported at heart level and
with the use of a properly calibrated and sized cuff. If the cuff is
too narrow or too short, readings may be erroneously high (typically
by 5 to 15 mm
Hg in the case of systolic pressure). The patient
should be asked whether he or she has smoked a cigarette within the
previous 15 to 30 minutes, since smoking can cause an elevation in
systolic blood pressure of 5 to 20
mm Hg. Avoidance of coffee is also
recommended, although the increase in systolic blood pressure after
one cup of caffeinated coffee is usually only 1 to 2 mm Hg. Long-term smoking or
coffee drinking does not cause persistently elevated blood pressure.
The diagnosis is
based on the findings of at least two or three elevated
blood-pressure measurements (in the physician's office or at home),
despite adherence to regimens containing three medications. However,
if the blood pressure is above 160/100 mm Hg, additional readings
are not necessary for diagnosis. Evaluation (including physical
examination and laboratory testing) is routinely warranted to look
for evidence of end-organ damage related to hypertension (Table 1) and for other cardiovascular risk factors.1 Volume overload and elevated sympathetic tone, which
are common in patients with uncontrolled blood pressure, may
occasionally be suggested by the presence of a rapid pulse rate. Renin levels have not been found
to be useful in the prediction of excess volume, though they may be
useful in the evaluation of possible secondary causes of hypertension.
 Some patients
who have what appears to be resistant hypertension
have a normal blood pressure at home. This phenomenon has been attributed
to transitory, or "white-coat," resistant hypertension
in the physician's office. Repeated home measurements or 24-hour ambulatory
monitoring may differentiate this type of hypertension from
truly resistant hypertension.13 Such measures are warranted in
patients undergoing treatment who have consistently high blood-pressure
levels in the physician's office yet have no evidence of
target-organ damage. In one study, as many as a third of patients
with apparently resistant hypertension had
average blood-pressure levels of less than 130/85 mm Hg on 24-hour or
home measurement.14 Some data suggest that blood-pressure values obtained at
home or during 24-hour ambulatory procedures correlate better with
target-organ involvement, especially left ventricular hypertrophy,
than do values obtained in the physician's office.15 However, office, or white-coat, hypertension
is not benign and should not be ignored.
Some patients
who have what appears to be resistant hypertension
have a normal blood pressure at home. This phenomenon has been attributed
to transitory, or "white-coat," resistant hypertension
in the physician's office. Repeated home measurements or 24-hour ambulatory
monitoring may differentiate this type of hypertension from
truly resistant hypertension.13 Such measures are warranted in
patients undergoing treatment who have consistently high blood-pressure
levels in the physician's office yet have no evidence of
target-organ damage. In one study, as many as a third of patients
with apparently resistant hypertension had
average blood-pressure levels of less than 130/85 mm Hg on 24-hour or
home measurement.14 Some data suggest that blood-pressure values obtained at
home or during 24-hour ambulatory procedures correlate better with
target-organ involvement, especially left ventricular hypertrophy,
than do values obtained in the physician's office.15 However, office, or white-coat, hypertension
is not benign and should not be ignored.
Rarely, in older
patients, what appears to be refractory hypertension may
represent inaccurate measurement owing to severely sclerotic arteries
(i.e., pseudohypertension). The
condition is suggested if the radial pulse remains palpable despite
occlusion of the brachial artery by the cuff (the Osler maneuver),16 although this sign is not specific. The presence of this
condition can be confirmed by
intra-arterial blood-pressure measurement.
Adherence to Treatment
Because a diagnosis
of resistant hypertension requires a finding
of elevated blood pressure despite the use of adequate doses of
at least three medications, the patient's adherence to therapy and the
adequacy of the dose should be evaluated routinely.
Studies have reported that medication was not increased
in more than 50 percent of patients with poorly controlled hypertension despite
repeated office visits. Some patients take less than the prescribed
dose of medication for economic or other reasons. However, side
effects have not been found to be an important reason
for a lack of adherence to therapy but may contribute to nonadherence. Signs suggesting nonadherence
include missed office visits and a lack of physiological evidence of
therapy (e.g., rapid heart rate despite the prescription of
beta-blockers or verapamil), but nonadherence is often difficult to recognize or exclude
objectively.
Interfering or Exogenous Substances
Patients should be asked routinely about the use of medications or
other substances that can elevate blood pressure or antagonize the
effects of antihypertensive drugs. These substances include sympathomimetic drugs (e.g., ephedra,
phenylephrine, cocaine, and amphetamines),
herbal supplements (e.g., ginseng and yohimbine),
anabolic steroids, appetite suppressants, and erythropoietin, although
all these drugs probably account for less than 2 percent of cases of
resistant hypertension. Nonsteroidal
antiinflammatory drugs and
cyclooxygenase-2 inhibitors may raise both systolic and diastolic
blood pressure by several mm Hg. These agents impair the excretion
of sodium, which causes volume retention; they also inhibit the
production of local renal vasodilative prostaglandins;
the therapeutic action of angiotensin-converting–enzyme
(ACE) inhibitors and loop diuretics (but not calcium-channel blockers)
depends on the availability of these prostaglandins.19,20 Efforts should be made to discontinue such agents,
although if they are needed for another condition, antihypertensive
therapy may need to be modified.
An assessment of
dietary and lifestyle factors is also important. Excessive
alcohol use (more than three or four drinks per day) and a high
sodium intake (typically defined by a urinary sodium excretion of
more than 150 mmol per day) may contribute to resistant hypertension; the frequency
of salt sensitivity is increased among patients who are at least 60
years of age, patients who are black or obese, and patients with
renal impairment. Studies indicate that more than 40 percent
of patients with resistant
hypertension
are obese,23,24 and obese patients may require higher doses of antihypertensive
medications than do nonobese patients.
Evaluation of Secondary Hypertension
The possibility that
an underlying condition is causing hypertension
must also be considered; secondary hypertension
is often unmanageable until the underlying cause is treated.11 Among 4000 patients with resistant hypertension
who were evaluated during an 18-year period at one tertiary center, secondary causes were found in 10
percent of patients overall and in 17 percent of patients over the
age of 60 years.25
Chronic renal parenchymal disease, usually resulting from diabetic nephropathy
or hypertensive nephrosclerosis, may be the most
common cause of secondary hypertension.
Atherosclerotic renovascular disease,
which is particularly prevalent among elderly smokers, is another
possible cause. The presence of an abdominal bruit or hypokalemia or a recent increase in the severity of hypertension
may suggest the diagnosis of atherosclerotic renovascular
disease. Screening for renovascular
disease may be warranted if other causes of
resistant hypertension are not identified,
since angioplasty and stenting may improve
blood pressure. However, in cases of renovascular
hypertension caused
by atherosclerotic disease, blood pressure often remains high even
after intervention, in contrast to hypertension caused by the much less common fibromuscular dysplasia.
Table 2 summarizes features of and screening tests for these and
other causes of secondary hypertension,
such as primary aldosteronism (considered to be more common than previously recognized), pheochromocytoma, and sleep apnea
(recently recognized to be associated with refractory hypertension). Generally,
the decision to screen a patient for such disorders should depend on
suggestive findings on history taking, physical examination, or
basic laboratory testing. Interventions that are directed at these
disorders (e.g., surgery or aldosterone-antagonist
therapy for hyperaldosteronism, surgery for pheochromocytoma, and continuous positive airway
pressure for sleep apnea) may substantially
decrease, although not always normalize, blood pressure. A detailed
discussion of all the causes of secondary hypertension is available elsewhere.

Treatment
Patients should
routinely be encouraged to reduce their intake of sodium, lose
weight (if appropriate), engage in moderate exercise, and reduce
their intake of alcohol (to no more than two to three drinks per
day). The degree of blood-pressure lowering expected
with each of these approaches is often modest but clinically important
— for example, 2 to 8 mm
Hg with dietary sodium restriction (with a goal of urinary sodium
excretion of less than 100 mmol per day),
2 to 4 mm
Hg with reduced alcohol consumption, and 4 to 9 mm Hg with regular physical
activity (such as walking briskly for 30 to 45 minutes daily).
Adherence to therapy
may be increased by the initiation of a system
of follow-up reminders or telephone contacts. The involvement of
nurses or nurse practitioners, who may have more time than a
physician to discuss potential side effects of medications, has been shown to improve patients' control of their blood
pressure. The use of combination therapy (two medications in one
pill) may also improve adherence and, in some cases, may reduce the
cost of care.
Few data from
randomized trials are available to guide the choice of regimen for
patients whose blood pressure remains high even though they take
several medications; recommendations are based largely
on physiological principles and clinical experience. Because volume
overload is common among such patients, the most important
therapeutic maneuver is generally to add or increase
diuretic therapy; more than 60 percent of patients with resistant hypertension may have a response to
this approach. Thiazide diuretics are
effective in doses of 12.5 to 25.0 mg daily if renal function is
normal. Experience suggests that a daily dose of 25 to 50 mg will
result in a further decrease in blood pressure. If the glomerular filtration rate is below 30 to 50 ml
per minute (or the serum creatinine level is more
than 1.5 mg per deciliter), loop diuretics
should be used. Short-acting loop
diuretics, such as furosemide (at a dose of 20 to 80
mg daily) or bumetanide (at a dose of 0.5
to 2.0 mg daily), must be given two or three times per day.
Intermittent natriuresis with once-daily
drug administration may lead to reactive sodium retention mediated
by the renin–angiotensin
system, with consequent inadequate blood-pressure control.
Longer-acting diuretics such as torsemide
(at 2.5 to 5.0 mg) may be given once a day
but are more expensive.
A generally useful strategy is to combine agents from various classes,
each of which has one or more of the following effects: a reduction
in volume overload (diuretics and aldosterone
antagonists), a reduction in sympathetic overactivity
(beta-blockers), a decrease in vascular resistance (through the
inhibition of the renin–angiotensin
system with the use of ACE inhibitors or angiotensin-receptor
blockers), the promotion of smooth-muscle relaxation (dihydropyridine
calcium-channel blockers and alpha-blockers), and direct vasodilation (hydralazine
and minoxidil), although the latter are less well
tolerated. An additional
medication with a different mechanism of action from others the
patient is receiving may further lower the blood pressure or
overcome compensatory changes in blood-pressure elevation caused by
the first medication without increasing adverse effects. For
example, adding a beta-blocker or ACE inhibitor may counteract the
stimulation of the renin–angiotensin
system by diuretics.34
Some logical
combinations include a diuretic with an ACE inhibitor, a
beta-blocker, or an angiotensin-receptor blocker or
an ACE inhibitor with a calcium-channel blocker. Most patients with
resistant hypertension are already receiving
combinations of these agents. In these instances, it may be
necessary to increase the dose or the frequency of administration
from once to twice daily or to include an
additional drug, such as an aldosterone antagonist
if a patient is already receiving a diuretic, an ACE inhibitor,
and a calcium-channel blocker. Certain medications may be preferred
if the patient has coexisting illnesses (Table 1 of the Supplementary Appendix, which is available with the full text of this article at
www.nejm.org). For example, the addition of an angiotensin-receptor
blocker, a beta-blocker, or an aldosterone
antagonist would be reasonable if the drug is not
already being used in a patient with heart failure.
The Figure below summarizes one approach to the optimization of drug therapy
in patients with resistant
hypertension. There
are limited data to guide whether some agents should
be stopped before others are added if multiple drugs are
inadequate.

If resistant hypertension persists, patients can
augment their therapy with an agent from a different class of drugs. For
example, if the patient is receiving an angiotensin-converting–enzyme
(ACE) inhibitor or an angiotensin-receptor blocker
(ARB) plus a diuretic and a beta-blocker, a dihydropyridine
calcium-channel blocker can be added. If the patient
is receiving an ACE inhibitor or an ARB plus a diuretic and a dihydropyridine calcium-channel blocker, a beta-blocker can be added. The practitioner may consider adding an aldosterone antagonist to any of the combinations (but with
extreme caution if the patient is receiving an ACE inhibitor or an ARB because
of concern regarding hyperkalemia).
Combined alpha- and
beta-blockers (labetalol and carvedilol)
may improve blood-pressure control. Centrally acting agents —
for example, clonidine,  2-adrenergic blockers, reserpine (in
low doses), and direct vasodilators (hydralazine or,
in rare cases, minoxidil) — may be
necessary in some cases, as tolerated. With direct vasodilators,
concomitant high-dose beta-blockers (metoprolol
or atenolol) and loop diuretics (furosemide)
will be needed to counteract reflex tachycardia
and edema. Aside from the aldosterone antagonist spironolactone
and alpha-blockers, which have been shown
to reduce blood pressure in patients with resistant hypertension, data are lacking to
predict the magnitude of further blood-pressure reduction associated
with the addition of one of these other medications; clinical
experience suggests a decrease in systolic pressure of about 5 to 10 mm Hg.
2-adrenergic blockers, reserpine (in
low doses), and direct vasodilators (hydralazine or,
in rare cases, minoxidil) — may be
necessary in some cases, as tolerated. With direct vasodilators,
concomitant high-dose beta-blockers (metoprolol
or atenolol) and loop diuretics (furosemide)
will be needed to counteract reflex tachycardia
and edema. Aside from the aldosterone antagonist spironolactone
and alpha-blockers, which have been shown
to reduce blood pressure in patients with resistant hypertension, data are lacking to
predict the magnitude of further blood-pressure reduction associated
with the addition of one of these other medications; clinical
experience suggests a decrease in systolic pressure of about 5 to 10 mm Hg.
Referral to a hypertension specialist should be
considered in patients whose hypertension
is difficult to
control despite an assessment of adherence, dose, and other factors
that may exacerbate the condition — particularly if the use of the
above-mentioned combinations is not helpful. In truly refractory cases,
other combinations of medications may be considered. Combinations that have been studied include dual diuretic therapy
(spironolactone at a dose of 25 to 50 mg daily
plus a thiazide at a dose of 12.5 to 50 mg
daily or a loop diuretic), which has been associated with a
reduction in systolic blood pressure of 20 to 25 mm Hg and in diastolic
pressure of 10 to 12 mm
Hg (larger decreases than those obtained with the use of a single
diuretic)27; dual calcium-channel
blockers (a dihydropyridine, such as amlodipine, plus a nondihydropyridine),
which has been associated with a reduction in systolic blood
pressure of 6 mm
Hg and a reduction in diastolic pressure of 8 mm Hg, as compared with
nifedipine alone35; and a combination of an
ACE inhibitor and an angiotensin-receptor
blocker, which has been associated with a reduction in systolic
blood pressure of 5 to 6 mm
Hg, as compared with either agent alone.36
However, the
risks of such regimens must be considered (e.g., hyperkalemia
with the ACE inhibitor plus an angiotensin-receptor
blocker).
Guidelines
European Society of Hypertension - European Society of
Cardiology Guidelines
7th JNC (American) Guidelines
British Hypertension Society Guidelines IV
Guidelines synthesis
The Seventh
Report of the Joint National Committee on Prevention, Detection,
Evaluation, and Treatment of High Blood Pressure emphasizes the need
to consider secondary causes, improper blood-pressure measurement,
volume overload, competing substances, obesity, nonadherence
to treatment, inadequate doses or inappropriate combinations of
medications, and alcohol consumption as factors in resistant hypertension.1 These guidelines
do not include specific recommendations regarding when or how to
evaluate patients for specific secondary causes of resistant hypertension or for the
management of truly resistant
cases.
Areas of
Uncertainty
Several
questions require further investigation.37 The true
prevalence of resistant
hypertension remains
uncertain. More information is needed to
determine the optimal evaluation of patients for secondary hypertension, including indications
for screening for hyperaldosteronism,
which appears to be underdiagnosed. Data
from randomized trials are needed to improve the
treatment of patients whose blood pressure remains high while they
are receiving multiple agents. In such patients, the possible role
of new drugs, such as inhibitors of renin and
endothelin-1, requires evaluation.
Summary and
Recommendations
The patient
in the vignette has elevated blood pressure, despite taking three
medications, with evidence of target-organ injury (retinal arteriopathy and left ventricular hypertrophy). Careful
assessment of her adherence to therapy is warranted.
Such adherence may be improved by
addressing the reasons for nonadherence, such
as side effects or the cost of medications, or by arranging for more
frequent office visits or telephone contact. The ibuprofen she takes
for osteoarthritis should probably be discontinued,
since it may contribute to blood-pressure elevation, and be replaced
with acetaminophen. Weight loss and a restriction of dietary sodium
should be encouraged. Since volume overload is common in refractory hypertension, she could increase her
dose of diuretic (with repletion of potassium as needed). If these
interventions are ineffective, a different class of drug (e.g., a
calcium-channel blocker) could be added, and the
patient could be screened for renovascular
hypertension, even
though in patients with this condition, blood pressure may remain
elevated despite intervention. Regular follow-up is
warranted, with a goal of maintaining the blood pressure
below 140/90 mm Hg.
Hypertensive
Retinopathy
Hypertensive retinopathy is a
condition characterized by a spectrum of retinal vascular signs
in people with elevated blood pressure.1 The detection of hypertensive retinopathy with the use
of an ophthalmoscope has long been regarded as part of the
standard evaluation of persons with hypertension.2,3,4 This
clinical practice is supported by both previous5 and current6 reports of the Joint
National Committee on Prevention, Detection, Evaluation, and Treatment
of High Blood Pressure (JNC), which list retinopathy as one of
several markers of target-organ damage in hypertension. On the basis of the JNC criteria, the presence of retinopathy may be an
indication for initiating antihypertensive treatment, even in people
with stage 1 hypertension (blood pressure, 140 to 159/90 to 99 mm Hg) who have no other
evidence of target-organ damage.
Despite the
JNC recommendation, the clinical implications of hypertensive retinopathy are unclear. Many
physicians do not regularly perform an ophthalmoscopic
examination as part of the care they provide to hypertensive patients, nor do they
include retinal findings when making decisions about treatment. Furthermore,
there is no clear consensus regarding the classification of hypertensive
retinopathy or whether
a retinal examination is useful for risk stratification.
The evidence
in support of the JNC guidelines on retinal findings in hypertension
is based on earlier studies that may not have direct relevance to
current clinical practice.7,8,9,10 These studies
have several important limitations. First, because they involved
patients who had uncontrolled and untreated hypertension, generalization
to contemporary populations of patients with lower blood-pressure
levels may be problematic. Second, retinopathy
as defined in these studies was based on a
direct ophthalmoscopic examination. This
technique has been shown to be unreliable, with high rates of interobserver variability (20 to 42 percent) and
intraobserver variability (10 to 33 percent) when
used in persons with mild hypertension.11,12 Third,
although many earlier studies cite increased mortality among persons
with hypertensive
retinopathy,8,9,10 few studies
have demonstrated associations between hypertensive retinopathy and specific
cardiovascular outcomes (e.g., incident stroke and coronary heart
disease) or have adequately controlled for relevant confounding
factors (e.g., hyperlipidemia and
cigarette smoking). Thus, whether hypertensive retinopathy
predicts the risk of cardiovascular outcomes independently of other
risk indicators has not been examined until
recently. The purpose of this review is to appraise recent studies
(i.e., from 1990 onward) in regard to the pathophysiology, epidemiology, and
cardiovascular associations of hypertensive
retinopathy and
the evidence that supports its use for risk stratification in
persons with hypertension.
Historical
Context and Classification
Hypertensive retinopathy was first
described by Marcus Gunn in the 19th century in a series of
patients with hypertension and renal disease.7 The retinal
signs he observed included generalized and focal arteriolar
narrowing, arteriovenous nicking, flame-shaped and
blot-shaped retinal hemorrhages, cotton-wool spots,
and swelling of the optic disk (Figure 1, Figure 2, and Figure 3). In 1939,
Keith et al. showed that these signs of retinopathy were
predictive of death in patients with hypertension.10 The authors
described a widely used classification system that categorized these signs
into four groups of increasing severity.
Examples of Mild Hypertensive Retinopathy.Panel A shows arteriovenous nicking (black arrow) and focal narrowing
(white arrow). Panel B shows arteriovenous nicking (black arrows) and widening or
accentuation ("copper wiring") of the central light reflex of the
arterioles (white arrows).

Examples
of Moderate Hypertensive Retinopathy. Panel A shows
retinal hemorrhages (black arrows) and a cotton-wool
spot (white arrow). Panel B shows cotton-wool spots (white arrows) and arteriovenous nicking (black arrows).

Example of Malignant Hypertensive Retinopathy. Multiple cotton-wool spots (white arrows), retinal hemorrhages (black arrows), and swelling of the optic disk
are visible.

However,
several reviews of hypertensive
retinopathy since 1996
have questioned the usefulness of the classification system by
Keith et al. (subsequently modified by Scheie) and
its relevance to current clinical practice. The major criticisms of
the original and modified classifications are that they do not
enable the clinician to distinguish among low retinopathy grades (e.g., grade
1 signs are not easily distinguished from grade 2 signs) and that
the retinopathy grades
are not closely correlated with the severity of hypertension.
Furthermore, a detailed categorization of retinopathy into four grades does
not appear to be supported by retinal
studies with the use of fluorescein angiography.
Pathophysiology
The retinal
circulation undergoes a series of pathophysiological
changes in response to elevated blood pressure. In the initial, vasoconstrictive stage, there is vasospasm and an increase
in retinal arteriolar tone owing to local autoregulatory
mechanisms. This stage is seen clinically
as a generalized narrowing of the retinal arterioles. Persistently
elevated blood pressure leads to intimal
thickening, hyperplasia of the media wall, and hyaline degeneration
in the subsequent, sclerotic, stage. This stage corresponds to more
severe generalized and focal areas of arteriolar narrowing, changes
in the arteriolar and venular junctions
(i.e., arteriovenous nicking or nipping), and
alterations in the arteriolar light reflex (i.e., widening and
accentuation of the central light reflex, or "copper wiring").
This is followed by an exudative stage,
in which there is disruption of the blood–retina barrier, necrosis
of the smooth muscles and endothelial cells, exudation of blood and
lipids, and retinal ischemia. These changes are
manifested in the retina as microaneurysms,
hemorrhages, hard exudates, and cotton-wool
spots. Swelling of the optic disk may occur at this time and usually
indicates severely elevated blood pressure (i.e., malignant
hypertension). Because better methods for the control of blood
pressure are now available in the general population, malignant
hypertension is rarely seen. In contrast,
other retinal vascular complications of hypertension, such as macroaneurysms and branch-vein occlusions, are not uncommon in patients with chronically elevated blood
pressure. The stages of hypertensive
retinopathy described
here, however, may not be sequential. For example, signs of retinopathy
that reflect the exudative stage, such
as retinal hemorrhage or microaneurysm, may be seen in eyes that do not have
features of the sclerotic stage (e.g., arteriovenous
nicking). The exudative signs are
nonspecific, since they are seen in diabetes and
other conditions.
Epidemiology
Since 1990,
there have been seven population-based epidemiologic studies
(involving a total of 26,477 participants) of various
signs of hypertensive
retinopathy. In all
seven studies, retinal photographs were used to define
specific signs of retinopathy
without regard to a predetermined grading system. All of the studies
were conducted in the general community and
included persons with and those without a history of hypertension.
In general,
these studies show that signs of hypertensive
retinopathy can be reliably identified with a standardized examination
of photographs of the fundus.
Reproducibility was substantial for the grading of retinal hemorrhages and microaneurysms
(e.g.,  =0.80 to 0.99) and fair to moderate for the grading of arteriovenous nicking and focal arteriolar narrowing
(
=0.80 to 0.99) and fair to moderate for the grading of arteriovenous nicking and focal arteriolar narrowing
( =0.40 to 0.79). In four populations,
generalized arteriolar narrowing was estimated from
an assessment of retinal vessel diameters with the use of digitized
photographs. This technique appears to have substantial
reproducibility (i.e., the intraclass correlation
coefficient ranged from 0.80 to 0.99 in four studies).
=0.40 to 0.79). In four populations,
generalized arteriolar narrowing was estimated from
an assessment of retinal vessel diameters with the use of digitized
photographs. This technique appears to have substantial
reproducibility (i.e., the intraclass correlation
coefficient ranged from 0.80 to 0.99 in four studies).
On the basis of photographic grading, these epidemiologic studies
show that signs of hypertensive
retinopathy are common
in people 40 years of age or older, even in those without a history
of hypertension. Prevalence rates ranged from 2
to 15 percent for various signs of retinopathy, in contrast to the earlier
report from the Framingham Eye Study that found a prevalence of less
than 1 percent among participants who underwent an ophthalmoscopic examination with dilation. The higher rates
of prevalence in these more recent studies are probably due to
a higher sensitivity of photography, as compared with clinical ophthalmoscopy, for detecting certain signs of retinopathy. However, there have been no studies that have directly compared
the sensitivity or reliability of photography with that of ophthalmoscopy for the detection of hypertensive retinopathy, as there have been
for diabetic retinopathy.
A higher
prevalence of retinopathy
has been reported among black persons than
among whites, a difference that is explained in large part by the
higher levels of blood pressure among blacks. The racial variation
confirms the results of a previous population-based survey that used
direct ophthalmoscopy34 and suggests
that retinal examination may be particularly useful for risk
stratification among blacks. Variations in the prevalence of
specific signs of hypertensive
retinopathy according
to age and sex have not been consistently
demonstrated. There have been fewer studies of the incidence
of hypertensive retinopathy. Two studies
indicate that the incidence of various signs of retinopathy over a period of five to
seven years ranges from 6 to 10 percent.
Blood
Pressure
Numerous
studies have confirmed the strong association between the presence
of signs of hypertensive
retinopathy and
elevated blood pressure. Two studies have further
evaluated the effect of a history of elevated blood pressure on the
occurrence of specific retinal signs. In both studies, generalized
retinal arteriolar narrowing and arteriovenous nicking
were associated with an elevation in blood pressure that had been
documented six to eight years before the retinal assessment; the
studies were controlled for concurrent blood-pressure
levels. This association suggests that generalized narrowing and
arteriovenous nicking are markers of vascular damage
from chronic hypertension. In contrast, other signs (focal
arteriolar narrowing, retinal hemorrhages,
microaneurysms, and cotton-wool spots) were related to current but not previous blood-pressure
levels and may therefore be more indicative of the severity of
recent hypertension.
Furthermore,
the observation of signs of retinopathy
in people without a known history of hypertension suggests that
these signs may be markers of a prehypertensive state. For
example, generalized and focal narrowing of the retinal arterioles has
been shown to predict the risk of hypertension in normotensive
persons.38 Other
factors unrelated to hypertension (e.g., hyperglycemia,
inflammation, and endothelial dysfunction24) may also be
involved in the pathogenesis of retinopathy.
The Risk of
Stroke
The strongest
evidence of the usefulness of an evaluation of hypertensive retinopathy for risk stratification is based on its association with stroke. It is well known that the retinal circulation shares
anatomical, physiological, and embryologic features with the
cerebral circulation. An autopsy study of patients with stroke
showed a close correlation between retinal and cerebral arteriolar
findings. Functional alterations in retinal blood flow in patients
with lacunar stroke have also been
reported.40
Epidemiologic
data from four large, population-based studies showed independent
associations between signs of hypertensive
retinopathy, as
defined by the findings on retinal photographs, and the risk of
stroke. The Atherosclerosis Risk in Communities study,
a multisite cohort study, showed that some signs of retinopathy (e.g.,
retinal hemorrhages, microaneurysms,
and cotton-wool spots) were associated with a risk of newly
diagnosed clinical stroke that was two to four times as high as that
for patients who did not have these signs, even when the analysis
was controlled for the effects of long-term elevations in blood
pressure, cigarette smoking, elevated lipid levels, and other risk
factors for stroke. This study has also shown that signs of retinopathy are associated with
reduced cognitive performance on standardized neuropsychological tests,
cerebral white-matter lesions, and cerebral atrophy as defined on the basis of findings on magnetic resonance imaging (MRI).
In the Atherosclerosis Risk in Communities study, the five-year relative
risk of stroke among participants who had both hypertensive retinopathy and cerebral lesions on MRI, as compared
with those who had neither of these findings, was 18.1 (95 percent
confidence interval, 5.9 to 55.4); among participants who had
white-matter lesions only, the relative risk of stroke was 3.4 (95
percent confidence interval, 1.5 to 7.7). This pattern
appears to reflect more severe or extensive subclinical cerebral microvascular disease in persons with both cerebral
and retinal markers of hypertensive
end-organ damage. In the Cardiovascular Health Study, after the
analysis was controlled for elevated blood pressure and risk
factors, persons with similar signs of retinopathy
(retinal hemorrhages, microaneurysms,
and cotton-wool spots) were twice as likely to have a history of
stroke as were those who did not have these signs (odds ratio, 2.0;
95 percent confidence interval, 1.1 to 3.6). Population-based
studies in Wisconsin and in Japanhave
shown that the risks of fatal and nonfatal stroke are two to three
times as high in persons with signs of retinopathy as they are in persons
who do not have these signs — an association that is independent of
cardiovascular risk factors.
These
population-based studies also show substantially weaker and less
consistent associations between other retinal changes (e.g.,
generalized and focal narrowing of the arterioles and arteriovenous nicking) and stroke, death from stroke, cognitive
impairment, and cerebral changes on MRI. The
retinal signs most strongly associated with stroke (i.e., retinal hemorrhages, microaneurysms, and
cotton-wool spots) are correlated with a
breakdown of the blood–retina barrier. The association of
these signs with stroke may therefore suggest that disruption of the
blood–brain barrier is a possible pathophysiological
feature in the development of cerebrovascular disease.
These findings also support the concept that an assessment of
specific signs, rather than the presence or absence of hypertensive retinopathy, may be important for
risk stratification.
The Risk of
Coronary Heart Disease
There are
fewer data regarding the association of hypertensive
retinopathy and
the risk of coronary heart disease. In the National Health
Examination Survey, persons with retinal arteriolar narrowing, as
detected on ophthalmoscopy, were two to six times as
likely to have preexisting coronary heart
disease as those without these changes, after the analysis was
controlled for the presence or absence of hypertension and diabetes
and for serum cholesterol levels. In a study of
560 men with hypertension and hyperlipidemia, the
presence of hypertensive
retinopathy predicted
a doubling of the risk of coronary heart disease (relative risk,
2.1; 95 percent confidence interval, 1.0 to 4.2), and the presence
of either generalized or focal narrowing of the arterioles predicted
almost a tripling of this risk (relative risk, 2.9; 95 percent confidence
interval, 1.3 to 6.2). In contrast, the Atherosclerosis Risk
in Communities study showed that generalized narrowing of the
retinal arterioles was associated with subsequent coronary heart
disease in women (relative risk, 2.2; 95 percent confidence interval,
1.0 to 4.6) but not in men (relative risk, 1.1; 95 percent
confidence interval, 0.7 to 1.8). This finding may reflect the
higher risk of coronary microvascular disease among
women than among men.
Treatment
Some
experimental studies and clinical trials have shown that signs of hypertensive retinopathy regress with the control
of blood pressure, although spontaneous resolution of these signs in
the presence of high blood pressure has also been reported.54 It is
unclear whether antihypertensive
medications that are thought to have direct beneficial effects on
the microvascular structure (e.g., angiotension-converting–enzyme inhibitors) would
reduce the damage of retinopathy
beyond the reduction effected by lowered blood pressure. In a small
study of 28 patients with mild hypertension who were randomly
assigned to receive treatment with enalapril
or hydrochlorothiazide, opacification of
the retinal arteriolar wall was significantly reduced after 26 weeks
of treatment with enalapril; no other signs of retinopathy were reduced.53 In contrast,
hydrochlorothiazide did not have any effect on the signs of retinopathy. However,
to date, there are no data from prospective, controlled trials that
demonstrate that the specific reduction of hypertensive retinopathy also reduces
the morbidity and mortality associated with cardiovascular disease.
It is also unclear whether the targeting of persons with hypertensive retinopathy for established
risk-reducing interventions offers additional advantages over the
use of strategies without regard to retinal findings.
Future
Research
The recent
data suggest that there are several lines of future research. First,
a standardized classification system for hypertensive
retinopathy should be developed that is relevant to contemporary clinical
situations and reflects the recent data. Evidence from recent
studies supports the development of a photographic classification system
that would be similar to the photographic grading of diabetic retinopathy. However, it is not yet
clear that retinal photography should be a routine part of the
management of hypertension or that photography is superior to ophthalmoscopy for the detection of signs of hypertensive retinopathy.
Second,
additional prospective studies are needed that
demonstrate independent associations of hypertensive retinopathy with various cardiovascular
outcomes. For example, there are no recent studies focusing on
whether signs of retinopathy
predict other hypertensive
complications, such as renal dysfunction or congestive heart failure.
It is also unclear whether a retinal examination would confer a
greater benefit in specific subgroups of populations (e.g., younger
people,45 women,49 and blacks33). An ongoing
longitudinal study involving a multiethnic population will provide further
insights into these issues.54
Third, it is
important to compare the relative value of a retinal assessment
(based on an ophthalmoscopic examination performed
with or without the use of photography) with other strategies of
risk stratification (e.g., the use of electrocardiography and
echocardiography). Finally, there is a need to evaluate whether
specific therapy that is focused on the retinal
microcirculation can reverse changes in retinopathy, and, if so, whether
this approach will also ultimately result in a reduced
cardiovascular risk.
The Clinical
Approach
How should
the physician use the available evidence? This review provides
compelling evidence that certain signs of hypertensive retinopathy are associated with an increased
cardiovascular risk that is independent of other risk factors. On
the basis of the strength of these reported associations, we propose
a simplified classification of hypertensive retinopathy
— none, mild, moderate, and malignant — according to the severity
of the retinal signs (Table 1). The
physician may continue to provide routine care for patients with no retinopathy, undertake more
vigilant monitoring of the cardiovascular risk in patients with mild
retinopathy (i.e., those
who have retinal arteriolar signs only), or adopt an aggressive
approach to risk reduction in patients with moderate retinopathy (Figure 4). The few
patients who have swelling of both optic disks and very high blood
pressure (i.e., malignant retinopathy)
need urgent antihypertensive
treatment. In hypertensive patients with swelling
of the optic disk, the physician should rule out anterior ischemic
optic neuropathy, which occurs more frequently than malignant hypertensive retinopathy and is typically manifested as unilateral disk swelling, visual
loss, and defects of the sectorial visual
fields.
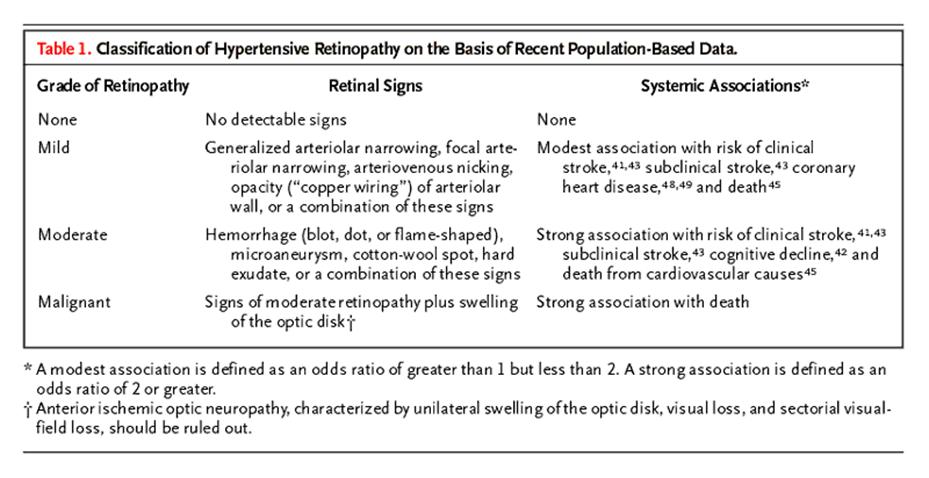

There is
insufficient evidence to recommend a routine ophthalmoscopic
consultation for all patients with hypertension. If the initial clinical
findings are equivocal (e.g., there is borderline or inconsistent
hypertension with no other evidence of target-organ damage), an ophthalmoscopic consultation may be useful to supplement
the risk assessment and treatment decisions. For some patients (e.g.,
those with diabetes or visual symptoms), referral may also be
important to rule out other conditions such as diabetic retinopathy or retinal-vein
occlusion.
Conclusions
Signs of hypertensive retinopathy are common and are correlated with elevated blood pressure.
Recent studies show that some of these signs (e.g., retinal hemorrhages, microaneurysms, and
cotton-wool spots) predict stroke and death from stroke independently
of elevated blood pressure and other risk factors. Patients with
these signs of retinopathy
may benefit from close monitoring of cerebrovascular
risk and intensive measures to reduce that risk.
Hypertensive
crises: urgencies and emergencies
Definitions
and probable causes of hypertensive crisis
Hypertensive urgencies
can be defined as severe elevations in BP that do not exhibit evidence of
target-organ (cardiovascular, renal, CNS) dysfunction or damage. Urgencies can be managed by the administration of oral medications,
most often in the emergency department (ED), and follow-up on an outpatient
basis. Hypertensive emergencies are severe elevations in systolic and
diastolic BP associated with acute target-organ damage that require immediate
management in a hospital setting.
Hypertensive
crises encompass a spectrum of clinical situations that have in common blood
pressure (BP) that is elevated, and progressive or
impending target organ damage. Most hypertensive urgencies or emergencies are
preventable and are the result of inadequate treatment of mild-to-moderate
hypertension or nonadherence to antihypertensive
therapy.
Some of examples of hypertensive crises
Traditionally,
hypertensive crises have been divided into emergencies
and urgencies. Hypertensive emergencies are severe elevations in blood pressure
(BP) that are complicated by evidence of progressive target organ dysfunction,
and will require immediate BP reduction (not necessarily to normal ranges) to
prevent or limit target organ damage. Examples include:
hypertensive encephalopathy, intracranial hemorrhage,
unstable angina pectoris, or acute myocardial infarction, acute left
ventricular failure with pulmonary edema, dissecting
aneurysm, or eclampsia. While the level of BP at the
time of presentation is usually very high (greater than 180/120 mm Hg), keep in
mind that it is not the degree of BP elevation, but rather the clinical status
of the patient that defines a hypertensive emergency. For example, a BP of
160/100 mm Hg in a 60-year-old patient who presents with acute pulmonary edema represents a true hypertensive emergency.
Hypertensive
urgencies are severe elevations of BP but without evidence of progressive target
organ dysfunction and would be better defined as severe elevations in BP
without acute, progressive target organ damage. A traditional
term “urgency” has led to aggressive and often excessive treatment of the
majority of patients who present to Emergency Departments (ED) with severe
hypertension. While these patients may present with levels of BP similar to the
hypertensive emergency, and may have evidence of target organ involvement, they
do not display evidence of ongoing progressive target organ damage. Most of
these patients are, in fact, nonadherent to drug
therapy or are inadequately treated hypertensive patients and often present to
the ED for other reasons. Patients with severe elevations of BP can be managed in the ED with oral agents and appropriate
follow-up within 24 hours to several days depending upon the individual
characteristics of the patient. It is the correct differentiation of these two
forms of hypertensive crises, however, that presents the greatest challenge to
the physician.
Initial
Assessment
A brief but thorough history should address the
duration as well as the severity of hypertension, all current medications
including prescription and nonprescription drugs and,
of particular importance, the use of recreational drugs. A history of other comorbid conditions and prior cardiovascular or renal
disease is essential to the initial evaluation. Direct questioning regarding
the level of compliance with current antihypertensive medications may establish
inadequacy of treatment or frank noncompliance.
Frequent or
continuous monitoring of BP should be established.
Look for historical information suggestive of neurologic,
cardiovascular, and/or renal symptoms. Check for specific manifestations such
as headache, seizures, chest pain, dyspnea, and edema. The clinical characteristics of a hypertensive
emergency are listed here. The level of BP alone does not
determine a hypertensive emergency; rather, it is the degree of target organ
involvement that will determine the rapidity with which BP should
be reduced to a safer level to prevent or limit target organ damage.
Initial therapy will be for a presumptive diagnosis based on the information
available during the initial triage evaluation.
The attached
algorithm (See algorithm) can help the
clinician identify those patients who meet the criteria of a hypertensive
emergency that requires immediate admission to an ICU for continuous monitoring
of BP and initiation of parenteral antihypertensive
therapy. For patients with uncontrolled hypertension (urgency), evidence of
target organ damage may or may not be present but these patients do not
demonstrate any evidence of deterioration of target organ function. They can be observed for several hours in the ED during which
time their oral medications can be resumed, if discontinued, or if untreated,
an oral regimen can be initiated. On occasion, increasing presently inadequate
dosages of medication may be appropriate. Appropriate outpatient follow-up can then be arranged within 24 hours to several days as
needed, and if no prior evaluation has been performed on this patient for
hypertension, an outpatient appointment should be established. Failure to
follow-up on this large group of patients is a missed opportunity from the
standpoint both of keeping patients in the healthcare system,
and establishing optimal BP.
Physical
Examination
The physical examination should begin with an assessment of BP, with an
appropriate-size cuff in both upper extremities and in a lower extremity if
peripheral pulses are markedly reduced. Brachial,
femoral, and carotid pulses should be assessed. A
careful cardiovascular examination as well as a thorough neurologic
examination, including mental status, should be conducted.
This assessment will aid in establishing the degree of involvement of affected
target organs and should provide clues to the possible existence of a secondary
form of hypertension, such as renovascular
hypertension. If a secondary cause of hypertension is
suspected, appropriate blood and urine samples should be obtained before
aggressive therapy is initiated. A careful funduscopic
examination should be performed to detect the presence
of hemorrhages, exudates, and/or papilledema.
Initial
Laboratory Studies
Initial laboratory studies should be limited and rapidly expedited. A
urinalysis with microscopic examination of the urinary sediment, an immediate
chemistry panel, and an electrocardiogram should be obtained.
The urinalysis may reveal significant proteinuria,
red blood cells, and/or cellular casts. Cellular casts are suggestive of renal
parenchyma disease. Electrolyte abnormalities, particularly hypokalemia
or hypomagnesemia, increase the risk of cardiac
arrhythmias, and the chemistry panel will also provide
evidence of renal and/or hepatic dysfunction. The electrocardiogram should
identify evidence of coronary ischemia and/or left ventricular hypertrophy and
may reveal pulse deficits, raising the question of aortic dissection. When the
clinical examination suggests cerebrovascular
ischemia or hemorrhage, or if the patient is
comatose, a computed tomographic scan of the head should be immediately obtained.
Initial
Treatment of the Hypertensive Emergency
The initial goal for BP reduction is not to obtain a normal BP but rather to
achieve a progressive, controlled reduction in BP to minimize the risk of hypoperfusion in cerebral, coronary, and renovascular beds. Under normal conditions, blood flow to
these organs remains relatively constant despite wide fluctuations in BP. In
the presence of severe hypertension, the autoregulatory
range is shifted upward so that higher levels of BP
are tolerated, but organ circulation may be put at risk with sudden reductions
in BP. As an example, studies on the autoregulation
of cerebral blood flow suggest that the lower limit of autoregulation
is about 25% below the resting mean arterial pressure in normotensive
subjects and in those with uncomplicated essential hypertension. These
observations have led to the suggestion that initial reduction in mean arterial
pressure should not exceed 20% to 25% below the pretreatment
BP. As an alternative, mean arterial pressure can be reduced
within the first 30 to 60 minutes to 110 to 115 mm Hg. If this level of
BP is well tolerated and the patient is clinically
stable, further gradual reductions toward a normal BP can be implemented over
the next 24 hours. Excessively rapid reductions in BP have been associated with
acute deterioration in renal function, ischemic cardiac or cerebral events, and
occasional retinal arterial occlusion and acute blindness.
A significant
exception to the above recommendations should be recognized for patients with
ischemic stroke, with the awareness that cerebral autoregulation
is disrupted in ischemic tissue. There is no clear evidence from clinical
trials to support the use of antihypertensive treatment during an acute stroke
in the absence of other concurrent disorders such as aortic dissection or heart
failure. Antihypertensive treatment may adversely affect cerebral autoregulation in acute stroke. Hypertension associated
with an acute ischemic stroke spontaneously decreases to pre-stroke levels
within several days.
How Urgent is Urgent Hypertension?
Historically, most patients seen in the ED with severe hypertension did not
meet the criteria for hypertensive emergency and were
therefore classified as having hypertensive urgencies. Most were treated aggressively in the ED and many were, in fact,
admitted to the hospital for control of BP. The important caveat is that
elevated blood pressure alone rarely requires emergency therapy. Initial triage
should identify those patients who have an elevated BP without any evidence of
significant target organ damage or any other impending cardiovascular events,
and these patients clearly represent the majority of those seen in the ED. They
are often asymptomatic and can be observed for a brief
period in the ED to initiate or resume medication in the noncompliant patient
or to increase dosages for those being inadequately treated. Follow-up can be arranged within several days in the outpatient
department.
The occasional
patient who presents to the ED with uncontrolled hypertension and symptoms such
as headache, shortness of breath, or epistaxis, may
benefit from observation in the ED over a period of several hours and/or an
increase in current medications or added medication to
further lower BP under observation and monitoring of current symptoms.
When clinically stable, the patient may safely be sent
home with oral agents and arrangements for follow-up. There
are several oral agents available that can provide rapid response in
blood pressure within one to several hours. These include agents such as the
short-acting ACE inhibitor, captopril, clonidine, Labetalol, or in
selected patients the alpha-adrenergic blocking agent prazosin.
Some of the pharmacologic characteristics of these oral agents are listed below.
In either case, to
discharge the patient from an ED without a confirmed follow-up appointment
represents a missed opportunity to get that patient back into treatment for
optimal control of BP, which should be a management goal.
There is little justification today to admit patients with hypertensive urgency
or high BP to a hospital for further evaluation and management when these
issues can be efficiently and cost-effectively addressed
in the outpatient setting.
Oral Agents
for Severe Hypertensive
Several oral agents can be particularly appropriate
for treating a hypertensive urgency in the ED.
Captopril,
an angiotensin-converting enzyme inhibitor, is well tolerated
and can effectively reduce BP in a hypertensive
urgency. Given by mouth, captopril is usually
effective within 15 to 30 minutes and may be repeated
in 1 to 2 hours, depending on the response. The drug has been
administered sublingually, in which case the onset of action is within
10 to 20 minutes, with a maximal effect reached within 1 hour. Responsiveness
to captopril can be enhanced
by the coadministration of a loop diuretic such as furosemide. Administration may lead to acute renal failure
in patients with high-grade bilateral renal artery stenosis,
and some reflex tachycardia may be observed.
Clonidine
is a centrally acting alpha-adrenergic agonist with onset of action 30 to 60
minutes after oral administration, and maximal effects are
usually seen within 2 to 4 hours. This agent is most
commonly administered as a loading dose of 0.1 or 0.2 mg followed by 0.1
mg hourly for several hours until an appropriate BP level is attained. Evidence
suggests that a comparable response may be seen with a
single 0.2 mg dose.5 The most common
adverse effect in the acute setting is drowsiness, affecting up to 45% of
patients. Clonidine may be a poor choice when
monitoring of mental status is important. Dry mouth is a common complaint, and
light-headedness is occasionally observed.
Labetalol,
a combined alpha- and beta-adrenergic blocking agent, can be
effectively administered orally in a dose of 200 to 400 mg with BP
response observed within 2 to 3 hours. However, the onset of effect may be observed within 1 to 2 hours. Like other
beta-blocking agents, labetalol has the potential to
induce heart block and to worsen the symptoms of bronchospasm
in the asthmatic patient. Caution must be observed in
patients who have more than first-degree heart block, symptomatic bradycardia, or congestive heart failure.
Prazosin
is an alpha-adrenergic blocking agent that can have limited benefit in the
early management of a patient with a pheochromocytoma.
Side effects include first-dose syncope, palpitations, tachycardia, and
orthostatic hypotension.
Parenteral Agents for Hypertensive Emergencies
See also Parenteral drugs for hypertensive emergencies
Parental therapy may be initiated in the ED if
suitable supervision and monitoring of
BP can be provided. More appropriately, the patient should be admitted to an
ICU where monitoring of BP is available. A number of parenteral
agents are effective in treating hypertensive emergencies (Table).
Labetalol
is effective when administered as 20- to 40-mg bolus
intravenous (IV) injections and can provide a step-wise, controlled reduction in
BP to a predetermined goal. Once the goal BP is achieved,
injections are stopped, and the long duration of action facilitates conversion
to effective oral therapy. A continuous infusion of labetalol
at 2 mg/min offers an alternative method of administration and is also associated with a gradual yet controlled reduction
in BP. Since the beta-blocking effects predominate with this agent, bradycardia or heart block may be
observed in patients with intrinsic heart disease.
Sodium nitroprusside has an extremely rapid onset
of action, within seconds of initiating an infusion, and a rapid offset of
effect within 1 to 2 minutes, which necessitates constant supervision of BP
during administration. This agent is particularly effective in reducing preload
and afterload in patients with impaired left
ventricular function, and a carefully titrated infusion can achieve any desired
goal BP. Nitroprusside does not cause sedation or
somnolence but is rapidly degraded by light, requiring
periodic exchange of solutions.
In patients with
significant renal impairment, accumulation of a major metabolite, thiocyanate, may occur over several days with toxic
effects. In the presence of poor tissue perfusion and depressed hepatic
function, an intermediate metabolite in the form of cyanide can accumulate and
occasionally lead to cyanide poisoning.
Nicardipine
is an IV form of the dihydropyridine calcium
antagonist and is effective in a high percentage of hypertensive emergencies,
particularly at higher infusion rates. Its growing popularity can be attributed to its ease of administration. Infusion
rates can be increased by 2.5 mg/hr at intervals of 15
to 20 minutes until the maximal recommended dosage of 15 mg/hr is obtained or
until the desired reduction in BP is achieved. Dosing of nicardipine
is not dependent on body weight. Nicardipine has been shown to reduce both cerebral and coronary ischemia
although headache, nausea, and vomiting may occasionally be observed.
Nitroglycerin
may be of particular benefit in hypertensive emergencies with coexisting
coronary ischemia. Since this agent dilates collateral coronary vessels and,
like nitroprusside, has a rapid onset and offset of
effect, its use requires close nursing supervision. Unfortunately, at low
infusion rates, nitroglycerin has its primary effect
on capacitance vessels in which much higher infusion rates are required to effect arteriolar vasodilitation.
Infusion rates may be increased at 3- to 5-minute
intervals until the desired effect is achieved. Nitroglycerin
may be particularly useful in patients with severe coronary ischemia whose BPs are only modestly elevated or in patients with acute
hypertension following postcoronary artery bypass
surgery. Tolerance to IV nitroglycerin may be observed within 24 to 48 hours of instituting an
infusion.
Fenoldapam
is a selective, peripheral dopamine1-receptor agonist that induces
systemic vasodilation, particularly in the renal
circulation.10
This agent also has effects on renal proximal and distal tubules. Fenoldapam does not bind to dopamine2 receptors
or beta-adrenergic receptors; it has no alpha-adrenergic agonist effects but is
an alpha1 antagonist. This drug does not cross the blood/brain
barrier.
Fenoldapam's unique effects on
the kidney provide increased urine flow rate, sodium and potassium excretion,
and improved creatinine clearance, making this agent
particularly attractive in hypertensive emergencies associated with significant
renal impairment.
Fenoldapam provides clinical
effects similar to those of nitroprusside in
improving cardiac hemodynamics in patients with acute
congestive heart failure. Onset of clinical effect is seen
within 5 minutes, and effects tend to dissipate within 30 minutes of
discontinuing the infusion. The most common side effects include headache,
flushing, tachycardia, and dizziness. A dose-related increase in intraocular
pressure has been observed in normotensive
and hypertensive patients. Inactive metabolites are
eliminated in the urine, and no dosage adjustments are required for
patients with renal or hepatic impairment.
Hydralazine
finds limited use today in pregnant women with preeclampsia. Five to 20 mg may
be administered IV as a bolus injection, and may be repeated. The major
advantage is this agent's ability to improve uterine blood flow. Hydralazine is contraindicated in
patients who have coronary atherosclerosis, as administration is associated
with reflex tachycardia and sodium and water retention, together with intense
flushing. Headache and increased intracranial pressure have
also been observed.
Other Parenteral Agents
Enalaprilat
is administered in an IV dose of 1.25 mg and may be
repeated at 6-hour intervals. Onset of action is within 30 minutes. Response to
enalaprilat in hypertensive emergencies is
unpredictable, in part because of variable degrees of plasma volume expansion.
This agent may be particularly suitable in hypertensive emergencies associated
with congestive heart failure or with high plasma angiotensin
II concentrations.
Esmolol
is an ultra-short-acting beta-adrenergic blocker that is
administered intravenously. Onset of effect is seen
within 1 to 5 minutes, with a rapid offset of effect within 15 to 30 minutes
after discontinuation. Esmolol may
be administered as a 500-µg/kg bolus injection, which may be repeated
after 5 minutes. Alternatively, an infusion of 50 to 100
µg/kg/min may be initiated and increased to 300 µg/kg/min as needed.
Adverse effects include increased heart block, precipitation of congestive
heart failure, and bronchial spasm.
Phentolamine
is a nonselective alpha-adrenergic blocking agent
that is still used when excess catecholamine states,
such as pheochromocytoma, are suspected. It is useful
as a diagnostic agent when administered as a bolus injection of 5 to 10 mg in
patients with suspected pheochromocytoma. Acute BP
lowering will be seen within several minutes and may
last 10 to 30 minutes. Tachycardia is common and on occasion may precipitate myocardial
ischemia. Nitroprusside and labetalol
are more easily titrated in the management of hypertensive emergencies
associated with high circulating levels of catecholamines,
and therefore phentolamine is
rarely used therapeutically today.
Diazoxide
is rarely used any longer in the treatment of
hypertensive emergencies. Although a potent vasodilator, large doses of 300 mg
were often associated with severe hypotension. Smaller miniboluses
of 50 mg administered at 10- to 15-minute intervals can provide controlled
reduction of BP but are usually associated with reflex tachycardia, hyperglycemia, hyperuricemia, and
sodium and water retention. In view of these side effects, diazoxide
offers little advantage over several other agents that have more acceptable adverse-effect
profiles.
Hypertensive
emergencies can carry a poor prognosis unless the BP is
reduced quickly, whereas hypertensive urgencies pose less immediate
danger. Hypertensive emergencies are considered to be clinical circumstances in
which the BP should be reduced immediately (within a matter of a few hours),
whereas in hypertensive urgencies, BP reduction can be accomplished gradually
(within several hours or even days). There is no arbitrary
level of BP that separates hypertensive emergencies from urgencies. The
presence of acute target-organ damage is more indicative of an emergency than an urgency. In either circumstance, the complications of
hypertensive crisis are reversible, if the treatment is
provided efficiently.3
Any type of
hypertensive disorder may be complicated by the
development of hypertensive crisis. The important proviso is that it is the
level of the BP—rather than the cause of hypertension—that is the main
determinant of hypertensive crisis.4 (In select forms of
hypertensive crises, however, the rapidity with which the BP rises seems to be
more relevant than the actual level of the BP.) In most clinical circumstances,
immediate BP reduction is indicated, not because of its absolute level but
because the coexisting complications may make any level of hypertension risky.
The risk-benefit ratio of immediate therapy in many forms of hypertensive
crisis is not well-established due to the paucity of
controlled clinical trials.
The most
critical decision in the management of hypertensive emergencies is to assess
the patient's clinical state and to ascertain whether the patient's condition
dictates emergency management. The absolute indications for treatment and
optimal management depend on the underlying and concomitant conditions. A patient
with a true hypertensive crisis should be treated in
an ICU.
The choice of
oral versus parenteral drug therapy depends on the
urgency of the situation, as well as on the patient's general condition. The
level to which the BP should be lowered varies with
the type of hypertensive crisis and should be individualized. The choice of parenteral drug is governed by the clinical manifestations
and concomitant medical problems associated with hypertensive crisis. There is
no predetermined level for the goal of therapy.
Complications
of therapy, mainly hypotension and ischemic brain damage, can occur in patients
given multiple potent antihypertensive drugs in large doses without adequate
monitoring.5 Such complications should be minimized by gentle
lowering of BP, careful surveillance, and individualization of therapy.
A relatively
asymptomatic patient who presents with severe hypertension, (a diastolic BP of
130-140 mm
Hg), need not be treated with parenteral drugs. These
patients should be managed on an individual basis, and
the usual course would be to intensify or alter the previous antihypertensive
therapy.
Once the
hypertensive crisis is resolved and the patient's clinical condition is stable,
the physician should look into factors that might have contributed to the
dangerous elevation of BP (nonadherence to prescribed
therapy or the presence and/or progression of a secondary form of hypertension
such as a renal artery stenosis or renal failure, for
example). The physician should discuss long-range and periodic outpatient
follow-up plans with the patient, as close follow-up is extremely important.
Accelerated
and malignant hypertension Accelerated hypertension is
clinically identified by severe retinopathy (without papilledema),
exudates, hemorrhages, arteriolar narrowing, and
spasm. Malignant hypertension is considered a
deterioration of the accelerated form and is distinguished by papilledema. Both the accelerated and malignant forms of
hypertension are associated with severe vascular injury to the kidney and other
target organs. The diagnosis of accelerated or malignant hypertension is best
made clinically on the basis of history and clinical
examination. Simple investigations such as chest radiography, ECG, urinalysis,
and laboratory tests to determine blood count, serum nitrogen level, creatinine clearance, and electrolyte concentrations are
generally sufficient.
The BP level
in malignant hypertension is often quite high, with diastolic levels in the
range of 130 to 140 mm
Hg (stage 2 hypertension), but the degree of BP elevation is an unreliable
diagnostic feature (see Table 2). Rather, it is the degree and extent of
vascular injury that determines the clinical manifestations. Severe headache,
with or without coexisting encephalopathy, is one symptom. Weight loss may
occur in some patients with malignant hypertension as a
result of initial natriuresis.
Some patients
with malignant hypertension report visual problems ranging from blurring to blindness;
drowsiness and altered mental status are also common. Worsening symptoms may
indicate progression to encephalopathy or other cerebral complications, such as
stroke. Congestive heart failure can be a feature of malignant hypertension,
either as a consequence of left ventricular
dysfunction, or due to volume retention from concomitant renal failure. Azotemia, a frequent feature of malignant hypertension, may
be associated with proteinuria. Renal function
deteriorates rapidly in many patients without appropriate therapeutic
intervention.
Patients with
accelerated/malignant hypertension should ideally be treated in the hospital,
since the goal of management is not only to lower the BP but
to stabilize it, reverse the target-organ damage, and exclude any reversible
causes. Preferably, these patients should be treated
in an ICU; but in the absence of significant target-organ dysfunction, they can
be managed safely on the wards under supervision.
Although IV
therapy is widely used in the initial treatment of malignant hypertension,
various oral therapies can also be used successfully.
ACE inhibitors, minoxidil, clonidine,
prazosin, labetalol, and nifedipine have all been used for
initial treatment of malignant hypertension, but there are no controlled
prospective studies to offer precise guidelines on the preferred therapeutic
options. The choice between oral and parenteral
therapy depends on the monitoring facilities, condition of the patient, and
coexisting complications. Once the BP is brought to safe levels, appropriate
oral therapy must be initiated on the basis of the
patient's renal, cardiac, and neurologic status.
Hypertensive encephalopathy is a deadly complication of severe hypertension that should be recognized as an emergency and quickly treated.
Although encephalopathy occurs mainly in patients with chronic or malignant
hypertension, it can also complicate sudden hypertension of brief duration. The
clinical manifestations are generated not only by the
severity of BP elevation but also by the abrupt rise of BP in a previously normotensive individual. This condition occurs more
frequently when the hypertension is complicated by
renal insufficiency than when the renal function is normal. The full-blown
clinical syndrome of hypertensive encephalopathy may take anywhere from 12 to
48 hours to develop.
Severe
generalized, sudden headache is a prominent clinical manifestation. Neurogenic symptoms consisting of confusion, somnolence,
and stupor may appear simultaneous with or following the onset of headache. If
untreated, progressive worsening of neurological damage occurs, culminating in
coma and death. The patient may be restless and uncooperative during the
initial stages of the syndrome. Other clinical features may include projectile
vomiting, visual disturbances ranging from blurring to frank blindness, and
transient focal neurologic deficits. Sometimes (especially in children)
generalized or focal seizures may be the only clinical feature.
On physical
examination of the patient, the BP is markedly elevated but there
is no certain level of BP above which encephalopathy is likely to develop.
The fundi reveal generalized arteriolar spasm with
exudates or hemorrhages. Although papilledema
is present in most patients with this complication, its absence does not
exclude the diagnosis of hypertensive encephalopathy.
When a
patient with uncontrolled hypertension presents with severe headache,
fluctuating mental status, papilledema, and variable
neurologic deficits, the most likely initial diagnosis should be hypertensive
encephalopathy. This must be distinguished from other
acute neurologic complications of hypertension such as cerebral infarction or hemorrhage and uremic encephalopathy. A complete but quick
evaluation of the patient should be carried out to
consider various diagnostic possibilities. The only definitive clue to confirm
the diagnosis of hypertensive encephalopathy is the prompt response of the
patient's BP reduction. The syndrome should improve or reverse within a few
hours with immediate control of the hypertension.
Once the
diagnosis of hypertensive encephalopathy is likely, the BP should be lowered
rapidly to near-normal levels; yet the diastolic BP should probably remain at
or slightly above 100 mm
Hg. Rapid reduction in the BP produces prompt, dramatic, and significant relief
of the symptoms of hypertensive encephalopathy. The most important goal of
therapy is to prevent permanent neurologic damage. Although potent orally
effective agents like minoxidil and nifedipine can control severe hypertension, parenteral drugs are preferred in treating hypertensive
encephalopathy successfully.1,6
Severe
hypertension and stroke pose a difficult dilemma. When intracerebral pressure rises as a result
of hemorrhage or thrombotic infarction, cerebral
blood flow may no longer be under normal autoregulation.
Therefore, a reduction in the systemic BP may conceivably compromise the
cerebral flow. Conversely, persistence of severe hypertension may resolve
spontaneously within 48 hours. There are no definitive data in the published
literature that provide the practicing physician with a definitive approach or
guidelines in managing these patients. At times it is
difficult to make a distinction between an ischemic stroke and an intracerebral bleed.
With the
current state of our knowledge, no reliable guidelines can be
given for the management of hypertensive crises occurring in patients
with cerebrovascular accidents. Based on the
pathogenesis of these conditions, especially intracerebral
hemorrhage, it is advisable to reduce the BP to
near-normal levels or to a degree that will not clinically compromise cerebral
function. If there is evidence of progression of the disease or worsening of
the neurologic manifestations during treatment, then the therapeutic approach must be reassessed. Precautions should be
taken to avoid hypotension in these patients, and it is advisable,
therefore, not to lower the diastolic BP to less than 100 mm Hg. In any case, the
BP reduction should be no more than the approximated 20% of the baseline BP
level.
Acute aortic
dissection has severe pain as its cardinal manifestation.7
This pain can easily be confused with the that of
other conditions, such as acute MI. There are certain subtle differences,
however: The pain of dissection is abrupt in onset, severe right from the
beginning, and unremitting in most patients, whereas patients with acute MI
rarely report abrupt onset of pain. The pain of MI may come and go, whereas the
pain of aortic dissection is continuous. Once the diagnosis is
suspected, immediate medical therapy should be implemented pending
further diagnostic evaluation.
Two-dimensional
echocardiography, transesophageal echocardiography,
CT, tomography, angiography, and magnetic resonance angiography (MRA) have all been used to diagnose aortic dissection. MRA
combined with transesophageal or transthoracic
echocardiography yields considerable information. However, MRA is recommended only for patients who are hemodynamically
stable. Digital and/or conventional angiography provides more
thorough information concerning the anatomy of dissection.
Once the
diagnosis of acute aortic dissection is apparent, the following steps should be
undertaken. If the patient is hypertensive, the BP should be
reduced to normal or below-normal levels with drugs that cause the BP to
come down smoothly rather than drastically. Direct vasodilators that
reflexively stimulate the heart should be avoided and
they are contraindicated in acute aortic dissection. When considering medical
therapy, keep in mind that the force and velocity of ventricular contraction (dP/dt) and the pulsatile flow are
the determinants of the shearing force acting on the aortic wall. Attempts should be made to decrease the dP/dt
with a suitable antihypertensive drug. Pharmacological options for acute aortic
dissection include labetalol, a combined alpha- and
beta-blocking drug, and the ultrashort-acting
beta-blocker esmolol, in combination with nitroprusside.
Acute left
ventricular failure may be caused by severe and uncontrolled hypertension; the higher the BP, the harder
the left ventricle must work. Decreasing the workload of the failing myocardium
may improve the heart function. In acute left ventricular failure, myocardial
oxygen requirements increase due to increased end-diastolic fiber
length and left ventricular volume. This could be particularly unfavorable in patients with concomitant coronary artery
disease (CAD).
Prompt
reduction of BP with a balanced vasodilating agent
such as nitroprusside is indicated.
Nitroprusside decreases both preload and afterload with restoration of myocardial function and
cardiac output. Although the ACE inhibitors, by the virtue of their
pharmacologic actions, may be useful in this situation, there is insufficient
clinical experience concerning acute therapeutic response to these agents in
patients with left ventricular failure. Morphine, diuretics, and other standard
drugs are also used in this setting.
Severe
hypertension associated with acute ischemic heart disease should benefit from BP reduction in theory, since systemic hypertension
increases myocardial oxygen consumption by increasing the left ventricular wall
tension. But there are no conclusive data to prove
that acute treatment is beneficial. Reduction of systemic BP reduces the
cardiac work, wall tension, and oxygen demand, and may thus limit myocardial
necrosis in the early phase of infarction. With a reduction in the afterload, the hemodynamic status improves significantly in
MI. Carefully supervised treatment of hypertension in patients with acute MI
is, therefore, likely to be beneficial.
Pheochromocytoma hypertensive crisis may manifest with
impressively dramatic clinical features. The BP is markedly elevated during the
paroxysm and the patient may have profound sweating, marked tachycardia,
pallor, numbness, tingling, and coldness of the feet and hands.8 A single attack may last from a few minutes to hours and may
occur as often as several times a day to once a month, or less.
If pheochromocytoma crisis is suspected,
the alpha-adrenergic blocking drug phentolamine (if
available) should be given in a dose of 1 to 5 mg IV, to be repeated within a
few minutes if needed. Nitroprusside would be an
alternative to phentolamine, but phentolamine
is more specific. A beta-blocking drug may be useful if the patient has a
concomitant cardiac arrhythmia. Administration of beta-blocking agents should always be preceded by either phentolamine
or pheno-oxybenzamine. Otherwise, beta blockade can
aggravate the unopposed alpha-mediated peripheral vasoconstriction. Labetalol has also been advocated
for this condition, but may not be generally effective in controlling the
clinical manifestations of pheochromocytoma.
Clonidine withdrawal syndrome can result from
abrupt discontinuation of a high-dosage regimen of clonidine,
causing a hyperadrenergic state that mimics pheochromocytoma. When clonidine is abruptly discontinued (especially at high dosages) or rapidly
tapered, a syndrome consisting of nausea, palpitation, anxiety,
sweating, nervousness, and headache, along with marked elevation of the BP has
been noted. Symptoms of clonidine withdrawal can be relieved by reinstituting the clonidine
regimen. If there is marked elevation of BP and the patient is experiencing
symptoms such as palpitations, chest discomfort, and epigastric
discomfort, the IV administration of phentolamine or labetalol is recommended.
Monoamine oxidase inhibitors (MAOIs) can increase the risk of hypertensive crisis in patients who also take
drugs such as ephedrine and amphetamines or consume foods containing large
quantities of tyramine. In the presence of an
inhibitor of monoamine oxidase, tyramine
and indirectly acting sympathetic amines escape oxidative degradation, enter
the systemic circulation, and potentiate the actions of catecholamines.
Sympathomimetic amines such as those contained in nonprescription cold remedies can also provoke this
response. Due to declining use of MAOIs, its reaction
is rare.
Cocaine-induced
hypertensive crisis can cause an abrupt, sudden increase
in the systemic BP, resulting in a hypertensive emergency. Neurohumoral
factors triggered by cocaine likely cause intense vasoconstriction and thus
increase the vascular resistance and the BP. Sudden rise of BP in a previously normotensive individual may result in a serious
cardiovascular complication. The BP should be lowered
to safe limits without much delay.
Eclampsia is a potentially serious
cardiovascular complication in a pregnant patient. Although the definitive
therapy is delivery of the fetus, the BP should be reduced to prevent neurologic, cardiac, and renal
damage. Although other antihypertensive drugs may be effective in reducing the
BP, the agent of choice is hydralazine, which has a
long record of safety. Animal studies have shown that nitroprusside
can cause problems in the fetus; therefore, its use should be reserved for hypertension refractory to hydralazine or methyldopa. The ganglion-blocking drug trimethaphan should be avoided
because of the risk of meconium ileus.
In pregnancy-induced hypertension, volume depletion may be present and
diuretics should be avoided. IV labetalol
and hydralazine have been used
to treat severe hypertension in pregnancy.9 ACE inhibitors and angiotensin-receptor blockers should be avoided due to
possible fetal/placental toxicity. Magnesium sulfate is also used as adjunctive
therapy to control the convulsions.
TREATMENT OF
A PATIENT WITH HYPERTENSIVE EMERGENCY
Whether or
not to hospitalize the patient, the therapeutic choices, and the choice of IV
versus oral therapy depend on the clinical evaluation of the patient and
available facilities. Patients with hypertensive emergencies should
be hospitalized, and those with hypertensive urgencies may not always
require admission to the hospital.
The
therapeutic premise underlying the management of hypertensive emergency is not
only to lower the BP quickly but to prevent, arrest,
and reverse the target-organ damage. Therefore, close supervision of the
patient is required while the BP is being lowered.
There are no firm guidelines as to the degree of desired BP reduction, but a
reasonable goal for most hypertensive emergencies is to lower the diastolic BP
to 100 mm
Hg (or to reduce the mean arterial pressure by 20%) over a period of minutes to
hours.
Hypertensive
emergencies should preferably be managed in an ICU to
allow for continuous monitoring of the general hemodynamic status of the
patient. Even though a secondary form of hypertension, such as renal artery stenosis or adrenal hypertension,
may be a causative factor, the immediate goal should be to lower the BP to a
safe level rather than undertaking a diagnostic workup. The rapidity of onset
and duration of action of the chosen drug are important considerations in
treating patients with hypertensive emergencies. The physician should be
familiar with the hemodynamic and pharmacologic actions of the available drugs.
Role of
concomitant diuretic therapy
Diuretics
have a limited role in the management of hypertensive emergencies; however,
they potentiate the therapeutic response of nondiuretic
agents. When the BP does not respond satisfactorily to an adequate dose of the
primary agent, adding a diuretic (such as furosemide)
may be helpful. In volume overload states such as
heart failure, concomitant administration of a loop diuretic is indicated for
optimal results. Diuretics should not be used
routinely in the management of hypertensive crises, as prior volume depletion
may be present in some conditions, such as malignant hypertension. The need for
diuretic therapy, therefore, should be individualized on the
basis of the hemodynamic and renal function status of the patient.
Orally
effective drugs
Clinical
experience has shown that antihypertensive drugs given orally as either single
or multiple doses can lower the BP immediately in patients with severe
hypertension. Obviously, this therapeutic opportunity is most suitable for
patients with hypertensive urgencies, not emergencies.
Nifedipine, a calcium channel blocker
given either orally or sublingually, has been shown to lower
the BP rapidly and can be useful in the management of hypertensive
crisis. Immediate reduction in BP can be accomplished
with sublingual (a punctured capsule, or nifedipine
liquid drawn out of the capsule with a syringe) or oral administration of the
capsules. The drug is also effective when the capsule is
bitten and then swallowed. The advantages of nifedipine
are rapid onset of action and lack of CNS depression. It may cause reflex
tachycardia. As the duration of action of nifedipine
is short, patients who receive this drug for hypertensive emergencies should be monitored for several hours to consider
re-administration of the drug. An abrupt fall in the BP induced by nifedipine administration can cause certain adverse effects—symptomatic
hypotension, tachycardia, and ischemic events—so the clinical need to use nifedipine capsules to lower the BP urgently should be carefully assessed and avoided, if possible.
Clonidine has been shown
to produce an immediate antihypertensive effect when given in repetitive doses.
Typically, clonidine loading was
accomplished in the ED by administering it orally at a dosage of 0.1 mg
q1h until the desired goal was obtained. The use of clonidine
loading therapy has declined due to the availability of safer and
better-tolerated alternative therapies that do not cause drowsiness.
Captopril is an ACE inhibitor that has been found to be effective in the immediate treatment of
severe hypertension and hypertensive crises. Captopril
lowers the BP promptly without causing tachycardia, and thus offers a distinct
hemodynamic advantage over direct arteriolar dilators. The maximal effect from
orally administered captopril may
not be attained for as long as 2 hours. There are some reports
documenting the effectiveness of sublingual captopril
in the treatment of hypertensive crisis, but further data needs to be generated to define its role in the acute management of
hypertensive crisis.
Minoxidil, a powerful direct vasodilator, has been successfully used in the treatment of refractory or
severe hypertension. Because of its relatively rapid onset of action and
sustained duration, this drug has been used for the
treatment of hypertensive crises. Minoxidil in
dosages ranging from 2.5 to 10 mg can be given every 4
to 6 hours initially in the treatment of severe hypertension. It works best
when given along with a diuretic, and an adrenergic blocker is necessary to
counteract the reflex tachycardia. Minoxidil should be used with caution in patients with acute coronary
syndromes unless the patient is beta-blocked.
Labetalol can be
administered orally in doses of 100 to 300 mg for
the treatment of hypertensive urgencies. Because of its dual adrenergic
blockade, the fall in BP is not accompanied by reflex
tachycardia. This attribute can be especially beneficial in patients with CAD.
Oral labetalol is effective within 1 to 3 hours and
may be useful for hypertensive urgencies, but it has an unpredictable dose
response and thus may not be ideal for acute situations. Labetalol
is contraindicated in patients with bronchospasm, heart block, significant bradycardia
or heart failure.
IV drugs for
hypertensive emergencies Several IV drugs are effective for use in hypertensive
crisis. The patient's clinical presentation will determine the agent of choice
in an individual situation. Parenteral drugs for
rapid control of severe hypertension are shown in
Table 3.
Nitroprusside is a powerful BP-lowering
drug that possesses the properties of rapid onset and offset of action. The hypotensive response occurs within seconds after the
infusion is started and disappears almost as rapidly
when the infusion is discontinued. The initial infusion rate should be 0.3
mcg/kg/min, which can be increased every 5 minutes
until a desired BP level is obtained. Once the desired effect is achieved, the BP should be continuously monitored.
Hypotension is the most common—but avoidable—adverse effect of nitroprusside therapy. Cyanide toxicity from nitroprusside is possible, although extremely rare.
Prophylactic infusion of cyanocobalamin (vitamin B12a)
25 mg/h, has been shown to decrease the cyanide concentration and tissue
hypoxia resulting from nitroprusside infusion during
surgery. Thiocyanate toxicity secondary to nitroprusside is uncommon and occurs only with high doses
and in the presence of renal failure. Treatment should be
interrupted when the thiocyanate level is
close to 10 mg/dL. Monitoring of plasma thiocyanate levels is not mandatory, as long as the
patient's clinical status is closely assessed. Treatment
of suspected thiocyanate toxicity requires
discontinuation of the drug and institution of dialysis.
Labetalol can be used
parenterally or orally for the treatment of
hypertensive emergencies. IV labetalol administered as either a continuous infusion or as bolus
injections reduces the BP promptly because of its rapid onset of action.
Controlled smooth reduction in BP may be obtained by
continuous infusion of labetalol at the rate of 0.5
to 2 mg/min. As with nitroprusside, close monitoring
of the patient is required during the infusion of labetalol.
Rapid (but not abrupt) lowering of BP can also be
accomplished with bolus injections of labetalol.
Labetalol should not be used
when beta-blockers are contraindicated—for example, in patients who may have heart
failure, atrioventricular block, asthma, or chronic
obstructive pulmonary disease.
Nicardipine is a dihydropyridine
calcium antagonist that exerts a prompt hemodynamic effect when given IV in
patients with severe hypertension. Nicardipine
infusion is started at 5 mg/h, and can be titrated upwards gradually to obtain
the desired therapeutic effect.11 Once a
stable BP is reached, most patients do not require further dosage alterations.
Thus, nicardipine pharmacodynamics
resemble those of nitroprusside
in terms of the onset, and duration and offset of action. Because of its
mechanism of action (calcium channel blockade), nicardipine
may be beneficial in preserving tissue perfusion. It is a good option in the
management of severe hypertension with or without target-organ damage.
Hydralazine has an antihypertensive action that
results from a direct relaxation of the vascular smooth muscle and it is accompanied by reflex increases in stroke volume and
heart rate, which can precipitate myocardial ischemia. Either IM or IV
administration of hydralazine causes an unpredictable
but definite fall in BP. In the treatment of hypertensive emergencies, the
initial IV dose should be 10 to 20 mg. The onset of the hypotensive
effect occurs within 10 to 30 minutes, and its duration of action ranges from 3 to 9 hours. The dose and frequency of administration
necessary to control the BP are highly variable. The delayed onset and
unpredictable degree of hypotensive effect present
difficulties in titration. Nevertheless, hydralazine
continues to be successfully utilized in the treatment
of eclampsia.
Phentolamine is an alpha receptor-blocking agent
that is specifically indicated for treating
hypertensive crises associated with increased circulating catecholamines.
These include, for example, pheochromocytoma crisis,
certain cases of clonidine withdrawal syndrome, and
crises resulting from an MAOI and drug-food interaction. The hypotensive effect of a single bolus injection is
short-lived and lasts less than 15 minutes. This drug may precipitate angina or
cardiac arrhythmias.
Nitroglycerin is a weak systemic arterial
dilator with a greater effect on large arteries than on smaller arteries. Low
doses cause venodilation; much higher doses are
required to produce a fall in systemic BP. Because of its pharmacologic
actions, nitroglycerin infusion may be particularly
beneficial in patients with CAD either with or without hypertension. Nitroglycerin improves coronary perfusion. Although there
are no controlled studies, nitroglycerin therapy can be considered in the management of severe hypertension
associated with CAD. The usual initial dosage of nitroglycerin
is 5 to 15 mcg/min, and the dosage is titrated upward to a desired therapeutic end point. It has a rapid onset (2-5 minutes) and offset of
action. Although nitroglycerin has
been used to achieve controlled hypotension, its main use continues to
be in patients with unstable angina and acute MI. Prolonged use may result in
tolerance. Isosorbide dinitrate
therapy has also been utilized for immediate treatment
of severe hypertension but its precise role and guidelines on how to use it are
not fully delineated.12
Enalaprilat, by its mechanism of action,
prevents conversion of angiotensin I to angiotensin II by blocking angiotensin converting
enzyme and thus lowering BP. Enalaprilat is the only
available parenteral ACE inhibitor. For hypertensive
emergencies, it is given at a dosage of 0.625 to 1.25
mg IV over 5 minutes and may be repeated every 6 hours. ACE inhibitors are contraindicated in patients with renal artery stenosis and in pregnant patients. ACE inhibitors can cause
precipitous falls in BP in patients who are hypovolemic.
These drugs are especially valuable in treating hypertensive emergencies in
patients with chronic heart failure.
Fenoldopam is a relatively new drug that is a
selective dopamine (DA1) receptor agonist and causes significant vasodilation. It is a short-acting, parenteral
arteriolar vasodilator that lowers BP by reducing peripheral vascular
resistance.13,14 By the mode of its action
on the DA1 receptor, fenoldopam causes
remarkable renal vasodilation and promotes diuresis and natriuresis. The
half-life of fenoldopam is
estimated to be 5 minutes, and the recommended starting dosage is 0.1
mcg/ kg/min. Its onset of action is 5 minutes and maximum response is achieved within 15 minutes. The dosage should be titrated
to gradually attain the goal BP. It can lower BP in
hypertensive emergencies safely and effectively and at the same time preserve
or improve renal blood flow and cause diuresis and natriuresis. Therefore, it may offer important advantages.
A number of early comparative studies between nitroprusside
and fenoldopam showed that while both are equally
effective in reducing the BP, fenoldopam offers
important renal advantages over nitroprusside.
References
1. Burt VL, Cutler JA, Higgins M, et al.
Trends in the prevalence, awareness, treatment and control of hypertension in
the adult US
population: data from the health examination surveys, 1960 to 1991. Hypertension
1995;26:60-69. [Erratum, Hypertension 1996;27:1192.] [Free Full Text]
2. The sixth report of the Joint National Committee on
Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med 1997;157:2413-2446. [Erratum, Arch Intern
Med 1998;158:573.] [Abstract]
3. Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind
placebo-controlled Systolic Hypertension in Europe
(Syst-Eur) trial. Lancet
1998;352:1347-1351. [CrossRef][ISI][Medline]
4. Kannel WB. Elevated systolic blood pressure as
a cardiovascular risk factor. Am J Cardiology 2000;15:251-5.
5. Staessen JA, Gasowski J, Wang JG, et al.
Risks of untreated and treated isolated systolic hypertension in the elderly:
meta-analysis of outcome trials. Lancet 2000;355:865-872.
[Erratum, Lancet 2001;357:724.] [CrossRef][ISI][Medline]
6. Stamler J. Blood pressure and high blood
pressure: aspects of risk. Hypertension 1991;18:Suppl I:I-95.
7. Perloff D, Grim CM, Flack J, et al. Human blood pressure
determination by sphygmomanometry. Circulation 1993;88:2460-2470. [Free Full Text]
8. Pickering TG. Recommendations for the use of home
(self) and ambulatory blood pressure monitoring. Am J Hypertens 1996;9:1-11. [CrossRef][ISI][Medline]
9. Kannel WB. Potency of vascular risk factors as
the basis for antihypertensive therapy. Eur Heart J 1992;13:Suppl G:34-42.
10. Dustan HP, Schneckloth RE,
Corcoran AC, Page IH. The effectiveness of long-term
treatment of malignant hypertension. Circulation
1958;18:644-651. [Free Full Text]
11. Collins R, Peto R, MacMahon S, et al. Blood
pressure, stroke, and coronary disease. 2. Short-term
reductions in blood pressure: overview of randomized drug trials in their
epidemiological context. Lancet
1990;335:827-838. [CrossRef][ISI][Medline]
12. Psaty BM, Smith
NL, Siscovick DS, et al. Health outcomes associated
with antihypertensive therapies used as first-line agents: a systematic review
and meta-analysis. JAMA 1997;277:739-745. [Abstract]
13. Appel LJ, Moore TJ, Obarzanek E,
et al. A clinical
trial of the effects of dietary patterns on blood pressure. N Engl J Med 1997;336:1117-1124. [Free Full Text]
14. Sacks FM, Svetkey LP,
Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the
Dietary Approaches to Stop Hypertension (DASH) diet. N Engl
J Med 2001;344:3-10. [Free Full Text]
15. Midgley JP, Matthew
AG, Greenwood CM, Logan AG. Effect of reduced dietary sodium
on blood pressure: a meta-analysis of randomized controlled trials. JAMA
1996;275:1590-1597. [Abstract]
16. Cutler JA, Follmann D, Allender PS. Randomized trials of sodium
reduction: an overview. Am J Clin Nutr
1997;65:Suppl:643S-651S. [Abstract]
17. Alderman MH, Madhavan S, Cohen H. Low urinary sodium is associated with
greater risk of myocardial infarction among treated hypertensive men. Hypertension 1995;25:1144-1152. [Free Full Text]
18. Kumanyika SK, Cutler
JA. Dietary sodium reduction: is there cause for concern? J Am Coll Nutr
1997;16:192-203. [Abstract]
19. Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar
S, Lacy CR. Effects of reduced sodium intake on hypertension control in older
individuals: results from the Trial of Nonpharmacologic
Interventions in the Elderly (TONE). Arch Intern Med 2001;161:685-693. [Free Full Text]
20. Neaton JD, Grimm
RHJ, Prineas RJ, et al. Treatment of Mild
Hypertension Study: final results. JAMA 1993;270:713-724. [Abstract]
21. Grimm RH Jr,
Grandits
GA, Cutler JA, et al.
Relationships of quality-of-life measures to long-term lifestyle and drug
treatment in the Treatment of Mild Hypertension Study. Arch Intern
Med 1997;157:638-648. [Abstract]
22. Hansson L, Zanchetti A, Carruthers
SG, et al. Effects of intensive blood-pressure lowering and low-dose
aspirin in patients with hypertension: principal results of the Hypertension
Optimal Treatment (HOT) randomized trial. Lancet 1998;351:1755-1762. [CrossRef][ISI][Medline]
23. UK Prospective Diabetes Study Group. Tight blood pressure control and
risk of macrovascular and microvascular
complications in type 2 diabetes: UKPDS 38. BMJ
1998;317:703-713. [Erratum, BMJ 1999;318:29.] [Free Full Text]
24. SHEP
Cooperative Research Group. Prevention of stroke by antihypertensive drug
treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly
Program (SHEP). JAMA 1991;265:3255-3264. [Abstract]
25. Materson BJ, Reda DJ, Cushman WC, et al. Single-drug therapy for
hypertension in men: a comparison of six antihypertensive agents with placebo.
N Engl J Med 1993;328:914-921.
[Erratum, N Engl J Med 1994;330:1689.] [Free Full Text]
26. Blood Pressure Lowering Treatment Trialists'
Collaboration. Effects of ACE inhibitors, calcium antagonists, and other
blood-pressure-lowering drugs: results of prospectively designed overviews of
randomised trials. Lancet
2000;356:1955-1964. [CrossRef][ISI][Medline]
27. The Heart
Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme
inhibitor, ramipril, on cardiovascular events in
high-risk patients. N Engl J Med 2000;342:145-153. [Erratum, N Engl J
Med 2000;342:748, 1376.] [Free Full Text]
28. Staessen JA, Wang JG, Thijs L.
Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet
2001;358:1305-1315. [Erratum, Lancet
2002;359:360.] [CrossRef][ISI][Medline]
29. Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff
SL, Gifford N, Schrier RW. The
effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with
non-insulin-dependent diabetes and hypertension. N Engl J Med
1998;338:645-652. [Free Full Text]
30. Hansson L, Lindholm LH, Ekbom T,
et al. Randomised trial of old and new
antihypertensive drugs in elderly patients: cardiovascular mortality and
morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet 1999;354:1751-1756. [CrossRef][ISI][Medline]
31. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint
reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet
2002;359:995-1003. [CrossRef][ISI][Medline]
32. Pahor M, Psaty BM, Alderman MH, et al. Health outcomes associated
with calcium antagonists compared with other first-line antihypertensive
therapies: a meta-analysis of randomised controlled trials. Lancet 2000;356:1949-1954. [CrossRef][ISI][Medline]
33. Major outcomes in high-risk hypertensive patients
randomized to angiotensin-converting enzyme inhibitor
or calcium channel blocker vs diuretic: the
Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial
(ALLHAT). JAMA 2002;288:2981-2997. [Free Full Text]
34. Wing LMH,
Reid CM, Ryan P, et al. A comparison of outcomes with angiotensin-converting-enzyme inhibitors and diuretics for
the treatment of hypertension in the elderly. N Engl J Med
2003;348:583-592. [Free Full Text]
35. Lewis EJ, Hunsicker
LG, Bain RP, Rhode RD.
The effect of angiotensin-converting-enzyme
inhibition on diabetic nephropathy. N Engl J
Med 1993;329:1456-1462. [Erratum, N Engl J Med 1993;330:152.] [Free Full Text]
36. Hostetter TH. Prevention of end-stage renal disease due to type 2 diabetes.
N
Engl J Med
2001;345:910-912. [Free Full Text]
37. Furberg CD, Psaty BM. Should calcium antagonists be
first-line agents in the treatment of cardiovascular disease? The public health perspective. Cardiovasc
Drugs Ther 1996;10:463-466. [ISI][Medline]
38. Saunders E, Weir MR, Kong BW, et al. A comparison of the efficacy and safety of a
B-blocker, a calcium-channel blocker, and a converting enzyme inhibitor in
hypertensive blacks. Arch Intern Med 1990;150:1707-1713. [Abstract]
39. Neutel JM, Smith DHG, Weber MA. Low dose combination therapy vs. high dose monotherapy
in the management of hypertension. J Clin Hypertens (Greenwich)
1999;1:79-86.
40. Prisant LM, Weir MR, Papademetriou
V, et al. Low-dose drug
combination therapy: an alternative first-line approach to hypertension
treatment. Am Heart J 1995;130:359-366. [ISI][Medline]
41. Guidelines Subcommittee of the World Health
Organization-International Society of Hypertension (WHO-ISH). 1999 World Health
Organization-International Society of Hypertension Guidelines for the
Management of Hypertension. J Hypertens
1999;17:151-183. [CrossRef][ISI][Medline]
42. Ramsay LE,
Williams B, Johnston GD, et al. British Hypertension Society guidelines for
hypertension management 1999: summary. BMJ 1999;319:630-635. [Free Full Text]
43. Jackson R, Barham P, Bills
J, et al. Management of raised blood pressure in New Zealand. BMJ 1993;307:107-110. [ISI][Medline]
44. Hoes AW, Grobbee DE,
Lubsen J. Does drug treatment improve survival? Reconciling the trials in mild-to-moderate hypertension. J Hypertens 1995;13:805-811. [ISI][Medline]
45. Vasan RS, Larson MG, Leip EP, et al.
Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001;345:1291-1297. [Free Full Text]
46. Mann SJ, Blumenfeld JD, Laragh JH. Issues, goals, and guidelines for choosing first-line and
combination antihypertensive drug therapy. In: Laragh
JH, Brenner BM, eds. Hypertension: pathophysiology,
diagnosis, and management, 2nd ed. Vol. 2. New York: Raven Press, 1995:2531-42.
![]() 140 mm Hg or
diastolic pressure
140 mm Hg or
diastolic pressure ![]() 90 mm Hg) is present in one in four adults in the United
States.1
The prevalence is higher among blacks
and older persons, especially older women. Table 1 shows the classification of
blood pressure according to the Joint National Committee on
Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.2 Hypertension is a risk
factor for stroke, myocardial infarction, renal failure, congestive
heart failure, progressive atherosclerosis, and dementia.3
Systolic pressure
is a stronger predictor of
cardiovascular events than is diastolic pressure,4 and isolated systolic hypertension, which
is common among older persons, is particularly hazardous.5
There is a
continuous, graded relation between blood pressure and the risk of cardiovascular disease;
the level and duration of
hypertension and the
presence or absence of
coexisting cardiovascular risk factors determine the outcome.6 Treatment of hypertension reduces the risk of stroke, coronary artery disease, and congestive
heart failure, as well as overall cardiovascular morbidity and mortality
from cardiovascular causes. However, only 54 percent of patients with hypertension receive treatment and only 28 percent
have adequately controlled blood pressure.1
90 mm Hg) is present in one in four adults in the United
States.1
The prevalence is higher among blacks
and older persons, especially older women. Table 1 shows the classification of
blood pressure according to the Joint National Committee on
Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.2 Hypertension is a risk
factor for stroke, myocardial infarction, renal failure, congestive
heart failure, progressive atherosclerosis, and dementia.3
Systolic pressure
is a stronger predictor of
cardiovascular events than is diastolic pressure,4 and isolated systolic hypertension, which
is common among older persons, is particularly hazardous.5
There is a
continuous, graded relation between blood pressure and the risk of cardiovascular disease;
the level and duration of
hypertension and the
presence or absence of
coexisting cardiovascular risk factors determine the outcome.6 Treatment of hypertension reduces the risk of stroke, coronary artery disease, and congestive
heart failure, as well as overall cardiovascular morbidity and mortality
from cardiovascular causes. However, only 54 percent of patients with hypertension receive treatment and only 28 percent
have adequately controlled blood pressure.1 





 Some patients
who have what appears to be resistant hypertension
have a normal blood pressure at home. This phenomenon has been attributed
to transitory, or "white-coat," resistant hypertension
in the physician's office. Repeated home measurements or 24-hour ambulatory
monitoring may differentiate this type of hypertension from
truly resistant hypertension.13 Such measures are warranted in
patients undergoing treatment who have consistently high blood-pressure
levels in the physician's office yet have no evidence of
target-organ damage. In one study, as many as a third of patients
with apparently resistant hypertension had
average blood-pressure levels of less than 130/85 mm Hg on 24-hour or
home measurement.14 Some data suggest that blood-pressure values obtained at
home or during 24-hour ambulatory procedures correlate better with
target-organ involvement, especially left ventricular hypertrophy,
than do values obtained in the physician's office.15 However, office, or white-coat, hypertension
is not benign and should not be ignored.
Some patients
who have what appears to be resistant hypertension
have a normal blood pressure at home. This phenomenon has been attributed
to transitory, or "white-coat," resistant hypertension
in the physician's office. Repeated home measurements or 24-hour ambulatory
monitoring may differentiate this type of hypertension from
truly resistant hypertension.13 Such measures are warranted in
patients undergoing treatment who have consistently high blood-pressure
levels in the physician's office yet have no evidence of
target-organ damage. In one study, as many as a third of patients
with apparently resistant hypertension had
average blood-pressure levels of less than 130/85 mm Hg on 24-hour or
home measurement.14 Some data suggest that blood-pressure values obtained at
home or during 24-hour ambulatory procedures correlate better with
target-organ involvement, especially left ventricular hypertrophy,
than do values obtained in the physician's office.15 However, office, or white-coat, hypertension
is not benign and should not be ignored. 
![]() 2-adrenergic blockers, reserpine (in
low doses), and direct vasodilators (hydralazine or,
in rare cases, minoxidil) — may be
necessary in some cases, as tolerated. With direct vasodilators,
concomitant high-dose beta-blockers (metoprolol
or atenolol) and loop diuretics (furosemide)
will be needed to counteract reflex tachycardia
and edema. Aside from the aldosterone antagonist spironolactone
and alpha-blockers, which have been shown
to reduce blood pressure in patients with resistant hypertension, data are lacking to
predict the magnitude of further blood-pressure reduction associated
with the addition of one of these other medications; clinical
experience suggests a decrease in systolic pressure of about 5 to
2-adrenergic blockers, reserpine (in
low doses), and direct vasodilators (hydralazine or,
in rare cases, minoxidil) — may be
necessary in some cases, as tolerated. With direct vasodilators,
concomitant high-dose beta-blockers (metoprolol
or atenolol) and loop diuretics (furosemide)
will be needed to counteract reflex tachycardia
and edema. Aside from the aldosterone antagonist spironolactone
and alpha-blockers, which have been shown
to reduce blood pressure in patients with resistant hypertension, data are lacking to
predict the magnitude of further blood-pressure reduction associated
with the addition of one of these other medications; clinical
experience suggests a decrease in systolic pressure of about 5 to 




