Methods
of examination and semiotics of urinary system in children.
Semiotics
of microscopic exchanges of urinary sedimentation (protein-, erythrocyte-,
leucocytesuria). Acute and chronic renal failure.
Nursing
the child with renal pathology.
Anatomy of the urinary system
The urinary system consists of paired kidneys with ureters, a urinary
bladder, and a urethra (Image 1). The kidneys are bean-shaped organs located in
the retroperitoneal space in the posterior aspect of the abdomen at each side
of the spinal column. A fibrous capsule contains each kidney and normally is
separable from the surface. In chronic renal disease the capsule adheres to the
kidney because of fibrosis. The kidneys lie in perinephric fat; the upper pole
of each kidney is at the level of the twelfth thoracic vertebra and the lower
pole at the level of the third lumbar vertebra.

Image 1. Anatomy of urinary system.
Table 1 lists the combined weight of kidneys at
different ages.
Table 1.
Mean combined
weight of both kidneys
at different ages
|
Age |
Weight, g |
Age, yrs. |
Weight, g |
|
0 Birth |
24 |
4 |
119 |
|
3 mos. |
41 |
6 |
140 |
|
6 mos. |
53 |
8 |
157 |
|
1 yrs. |
70 |
10 |
171 |
|
2 yrs. |
91 |
12 |
183 |
|
3 yrs. |
107 |
Adult |
300 |
The renal length correlates with age, body weight, and body length.
In the healthy term newborn it is around
The adrenal glands are located above each kidney, although tumor and hemorrhage
of the gland may displace the kidney downward. The contractions of the
diaphragm displace the kidneys downward during inspiration. In the anteromedial
aspect of each kidney there is a slit called the hilus, the site for the
entrance of the renal artery and nerves and the exit of the renal vein,
lymphatics, efferent nerves, and renal pelvis. The relatively large size of the
kidneys in the newborn period allows for palpation. Fetal lobulations in the kidneys of newborns are of no clinical consequence and disappear during
infancy.
On a bisected surface of a kidney, two distinctive areas are identifiable. There is a a pale inner area (the
medulla) and a darker superficial region (the renal cortex) with a thickness of about
The minor calyces form the major calyces. The upper collecting system consists of the calyces, the
renal pelvis, and the ureter. The walls of the upper collecting system contain
smooth muscle that contracts to help transport urine to the lower collecting system (bladder). Each ureter
originates in the lower
part of the renal pelvis at the level of the ureteropelvic junction (UPJ) and extends down
to the bladder, entering at the level of the superior angles of the trigone. The lower angle of the trigone is the opening of the bladder
neck.
Kidney components
Light microscopic examination of the kidney reveals components that constitute the substance of the
kidney, including nephrons, blood vessels, interstitial tissue, and nerves. The nephron is
the structural and functional unit of the kidney; its function
is urine formation (Image 2.).

Image 2. Cross section
of a kidney and single nephrone.
The components of a nephron include the glomerular
corpuscle, or glomerulus, a tuft of specialized capillaries surrounded by a
capsule (Bowman’s capsule); the proximal convoluted tubule,
originating from the tubular pole of Bowman’s capsule; followed by the loop of
Henle, the distal convoluted tubule, and the collecting duct. The glomeruli, the proximal
convoluted tubule, most of
the loop of Henle, the distal convoluted tubule, and the cortical collecting ducts
are located in the renal cortex. There are two populations of glomeruli, those with the long loop of Henle extending to the tip of the renal
papillae, found deep in the renal cortex adjacent to the outer medulla, and those more superficial in the renal cortex, possessing shorter loops
of Henle that also lie mainly in the renal cortex. The difference in the
length of the loop of Henle may have functional implications, specifically in the
ability of the kidneys to concentrate urine with preservation of volume.
Blood supply and lymphatic drainage
The blood enters the kidneys via the renal arteries
that originate from the aorta. Usually there is a single renal artery for each
kidney, but variations are frequent.
In the adult, 20 to 25 percent of cardiac output goes to the kidneys. The renal artery enters the renal sinus and divides
into anterior and posterior branches in the hilar
region. Three segmental arteries (superior, middle, and inferior) arise
from the anterior branch that supplies blood to the anterior half of the
kidney. The posterior branch provides blood to the posterior half of the
kidney, except for the upper pole, which receives blood from the anterior
branch.
There is no anastomosis
between the segmental arteries, and if a segmental artery occludes, the renal segment will
die.
The veins that drain
the kidney are not segmental. The left renal vein is longer
than the right and crosses posterior to the superior mesenteric artery and
anterior to the aorta, emptying into the inferior vena cava. The right
renal vein drains directly into the inferior vena cava. The lymphatic fluid in
the kidney drains into lymphatic vessels that pass through the renal
sinus and hilum to drain into the lumbar lymph nodes.
Innervation
of the kidney
The kidney receives sympathetic
fibers that originate in the spinal cord
(segments T8–L2) and synapse in the renal ganglia in the
renal plexus. Stimulation of these nerves causes vasoconstriction and
decreases blood flow to the kidneys. Sensory fibers travel the
sympathetic pathway to segments T10 and T11. Renal pain refers to the flank
regions within these dermatomes. Parasympathetic innervation to the kidneys is
unclear.
Innervation
to the ureter
The ureter receives
sympathetic fibers from the renal plexus and preaortic plexuses.
Visceral afferent fibers travel the sympathetic nerves, and ureteral
pain is referred to dermatomes T11–L2.
Innervation
of the bladder
The parasympathetic
innervations of the bladder consist of the pelvic splanchnic
nerves (S2–4) originating from the inferior hypogastric plexus. These
parasympathetic fibers innervate the detrusor muscle involved in reflex
bladder contraction during micturition. Sympathetic innervation of the
bladder is involved in urinary retention by inhibition of activity of the detrusor
muscle and increasing urethral resistance. Relaxation of the
external urethral sphincter and pubococcygeus muscle is necessary to
initiate micturition. Visceral afferents travel along the pelvic splanchnic nerves.
Renal microcirculation
The nephron is the basic
structural and functional unit of the kidney, with
between 800,000 and 1,200,000 nephrons occurring in each human kidney. It
consists of the renal corpuscle (glomerulus and Bowman’s capsule)
and the renal tubule. The renal tubule has several segments, including
the proximal convoluted tubule with its straight part, the loop of Henle
with its thin descending and ascending parts, the thick ascending segment of
the loop of Henle, the distal convoluted tubule, and the collecting
duct.
In order to understand
the function of the nephrons, it is necessary to
understand their relationship to renal microvasculature. Blood enters the kidney
via the interlobar arteries that are tertiary branches of the renal
arteries. The interlobar arteries travel between the renal pyramids and give off the arcuate arteries that travel along the
corticomedullary junction. The arcuate arteries give off small arteries,
the interlobular arteries, that ascend to the cortex. The afferent glomerular
arterioles branch off the interlobular arteries. The afferent
glomerular arterioles enter Bowman’s capsule, branching into the glomerular
capillaries that then drain into a portal vessel, the efferent glomerular
arteriole. The efferent arteriole then takes the blood to a second
capillary network called the peritubular capillaries.
Glomerular filtration
results from intraglomerular capillary pressure under the
influence of independent contraction and dilation of the afferent and
efferent glomerular arterioles. The peritubular capillaries are specialized vascular
structures that facilitate reabsorption of the renal interstitial fluid from
the renal cortex and renal medulla. A branch of the efferent
arteriole descends straight into the renal medulla. These terminal branches
of the efferent arterioles are the arteriolae rectae. These vessels enter the
peritubular capillary network at various levels; the peritubular capillaries
enter venules that ascend the medulla toward the cortex in mirror image of
the arterial side, the venae rectae. These vessels are collectively called the
vasa recta (vasae rectae) and act as a countercurrent exchange system
that helps to maintain the osmotic gradient in the renal medulla.
Changes in renal function at birth
During intrauterine life, the
maternal kidneys maintain fetal fluid volume, electrolyte,
and acid-base homeostasis. The placenta functions as a
dialyzer. The fetal kidneys contribute to the formation and maintenance of the
amniotic fluid volume. Agenesis of fetal kidneys or inability of the
kidneys to function during fetal life, for example, because of certain
medications, results in oligohydramnios and lung hypoplasia.
At birth, the renal
response to the new environment and success in maintaining homeostasis
correlates with gestational age, as well as events that have
taken place during intrauterine life. These include congenital malformations
and intrauterine growth retardation of diverse causes that may
permanently affect renal function. All newborn infants void during the
first 24 hours after birth regardless of their gestational age.
After the second day of
life, oliguria is urine flow of less than 1 ml/kg per hour.
Polyuria exists when the urinary output is more than 2000 ml/1.73 m2 per day
and needs further investigation. After delivery, renal blood flow
increases significantly owing to several factors, including a decline in
renal vascular resistances because of an increase in prostaglandin synthesis
and an increase in systemic arterial blood pressure.
Glomerular filtration rate
(GFR) doubles at the end of the first week of life in
term infants. Infants born before 34 to 35 weeks of gestation have a
slower rate of GFR increase owing to incomplete nephrogenesis; however,
it increases rapidly after the 35th week of gestation. In full-term
infants, the serum these reflects the mother’s creatinine level, and these
decrease by 50 percent at the end of the first week. In preterm
infants, the rate of decline of serum creatinine at birth is slower,
reflecting the stage of nephrogenesis. In children, GFR corrected for
surface area of
Development of the kidneys
The most common causes
of chronic kidney disease and the need for dialysis
and transplantation for infants, children, and adolescents up to 18 years
of age are congenital abnormalities or genetically determined renal and
urologic diseases. The general use of prenatal ultrasonographic evaluation
of pregnancies has resulted in prenatal findings of renal and
urologic abnormalities of clinical consequences. The physician at a
minimum must deal with this information and notify the parents of the
potential consequences of these findings. Therefore, a basic understanding of the
embryologic development of the kidneys and urinary tract is
essential.
The kidneys develop
from the intermediate mesoderm. In mammalians, kidneys
develop in intrauterine life as the pronephros, the mesonephros, and the metanephros.
The first evidence of the pronephros in humans
occurs at the end of the third gestational week and degenerates by the
fifth week. The earliest stage of development of the mesonephros in humans
is in the fourth week. It functions as a transient kidney, serving as an
excretory organ for the embryo. The mesonephric tubules lack the loop
of Henle, the macula densa, and the juxtaglomerular apparatus, and the
tubules open laterally into the mesonephric ducts, which connect to the
urogenital sinus. The mesonephros obtains its maximal size by 8 weeks
and regresses by 16 weeks with only some elements retained as parts
of the reproductive organs in the male. The metanephros, or definitive
kidney, originates from the interaction of the ureteric bud arising from the
lower end of the mesonephric duct at the fifth week as it enters the
urogenital sinus. The ureteric bud comes in contact with the metanephric
mesenchyme at the twenty-eighth day of gestation and begins
dichotomous divisions. The ureteric bud dilates at its growing tip, and this
area becomes the ampulla, which interacts with the metanephric mesenchyme,
forming a cap and inducing the formation of future nephrons.
This process gives the metanephros a lobulated appearance.
In humans,
nephrogenesis is complete by 34 to 35 weeks (238–245 days). Reciprocal
inductive influences of the ureteric bud and the metanephric mesenchyme
activate numerous genes sequentially. The formation of the
collecting system results from the initial few divisions of the ureteric bud, given
origin to the renal pelvis, major and minor calyces, and collecting ducts. By
the 6 to 9 weeks, the kidneys ascend from the pelvis to the
lumbar site below the adrenal glands.
Urine production in
the human fetal kidney begins between the tenth and
twelfth gestational week and increases significantly during the third
trimester. Urine volume is around 5 ml/h at 20 weeks of gestation and
increases up to 50 ml/h at 40 weeks. The fetal metanephric kidney has
a relatively low blood flow and GFR compared with the adult. The
normal fetal urine is hypotonic in relation to plasma because the fetal
kidney also conserves less sodium than the adult kidney. Fetal urine is
hypotonic and has a high sodium content and a large volume compared
with that of a term newborn. The evaluation of these parameters and
beta2-macroglobulin in fetal life is, on occasion, helpful to assess the
health of the kidneys in fetal life.
Renal function
1. To maintain
the composition and volume of the body fluids at a constant level.
2. Formation
of an ultrafiltration of plasma, with subsequent.
3. Reabsorption
of most of the water and electrolytes by the renal tubules.
4. Secretion
of certain other substances into the tubular urine.
5.
Reabsorption is the transport of a substance from the tubular lumen to the
blood in surrounding vessels.
6. Secretion
is transport in the opposite direction, that is, from the blood to the lumen.
7. The
production of certain humoral substances.
- an enzyme erythropoietic
stimulating factor (ESF, or erythrogenin), which acts on a plasma globulin to form
erythropoietin),
- renin, is also secreted by the kidney
in response to reduced blood volume, decreased blood pressure, or increased
secretion of catecholamines,
- renin stimulates the production of the angiotensins, which produce arteriolar
constriction and an elevation in blood pressure and stimulate the
production of aldosterone by the adrenal cortex.
Table 1.
Functions and dysfunctions of the kidney
|
Function |
Dysfunction |
|
1.
Salt, water and
acid-base balance |
|
|
Water Balance |
Fluid retention and Na |
|
Sodium Balance |
Edema, CHF, HTN |
|
Potassium Balance |
Hyperkalemia |
|
Bicarbonate Balance |
Metabolic acidosis, osteodystrophy |
|
Magnesium Balance |
Hypermagnesemia |
|
Phosphate Balance |
Hyperphosphatemia, osteodystrophy |
|
2.
Excretion of
nitrogenous and products |
|
|
Urea, creatinine, uric acid, amine, guanidine derivatives |
Anorexia, nausea, pruritis, pericarditis, polyneuropathy, encephalopathy, thrombocytopathy |
|
3.
Endocrine/Metabolic
function |
|
|
Synthesis of vitamin D |
Osteomalacia, osteodystrophy |
|
Production of erythropoietin |
Anemia |
|
Renin |
Hypertension |
Kidney function in
early infancy
1.
Glomerular filtration
rate is low and does not reach adult values until the child is between 1 and 2 years of age.
2.
There is a large variation
in the tubular length between nephrons, although
glomerular size is less variable.
3.
The juxtaglomerular nephrons show more advanced
development than cortical nephrons.
4.
The concentrating
ability of the newborn kidney does not reach adult
levels until about the third month of life.
5.
Adequate amounts
of antidiuretic hormone are secreted by the newborn pituitary gland, other
factors appear to interfere with water reabsorption.
6.
The Henle’s loop, essential to concentration ability, is
incompletely developed in the newborn.
7.
Urea synthesis
and excretion are slower during this time.
8.
The newborn
retains large quantities of nitrogen and essential electrolytes in order to
meet needs for growth in the first weeks of life.
9.
Consequently the
excretory burden is minimized.
10.
The lower concentration of urea, the principal end
product of nitrogen metabolism, reduces
concentrating capacity, since it also contributes to the concentration
mechanism.
11.
Newborn infants
are unable to excrete a water load at rates of older persons.
12.
Hydrogen ion
excretion is reduced.
13.
Acid secretion is
lower for the first year of life.
14.
Plasma
bicarbonate level is low.
15.
As a result of
these inadequacies of the kidney and less efficient
blood buffers, the newborn is more liable to develop severe acidosis.
16.
Sodium excretion
is reduced in the immediate newborn period, and the kidneys are less able to
adapt to deficiencies and excesses of sodium.
17.
An isotonic saline infusion may produce edema because the ability to eliminate excess sodium is
impaired. Conversely inadequate reabsorption of sodium from tubules may
compound sodium losses in disorders such as vomiting or diarrhea.
18.
Infants have a
diminished capacity to reabsorb glucose and, during the first few days, to
produce ammonium ions.
THE PHYSICAL
EXAMINATION OF THE URINARY SYSTEM
The primary symptoms of urinary
system disorders are pain and changes in the frequency of urination.
The nature and location of the pain
can provide clues to the source of the problem.
For example,
• Pain in the superior pubic region
may be associated with urinary bladder disorders.
• Pain in the superior lumbar
region or the flank that
radiates to the right upper quadrant or left upper quadrant can be caused by
kidney infections such as glomerulonephritis,
pyelonephritis, or
kidney stones.
Renal pain.
This is pain arising from the
kidneys and
• is usually felt at or below the
costal margin posteriorly
• may radiate anteriorly towards the
umbilicus
• is visceral pain produced by
distention of the renal capsule
• is typically dull aching and
steady.
Ureteric pain: Results from sudden distention of
the ureter and associated distention of the renal pelvis. It is severe colicky pain which originates in the
costovertebral angle.
It may radiate into the lower
quadrant of the abdomen and possibly to the upper thigh and testicle or labium.
Individuals with urinary system
disorders may urinate more or less frequently than usual and may produce normal or abnormal amounts
of urine:
• An irritation of the lining of
the ureters or urinary bladder
can lead to the desire to urinate with increased frequency, although the total amount of urine produced each day
remains normal. Detrusor muscle
contractions may also lead
to increased frequency in urination. When these problems exist, the individual feels the urge to urinate when the urinary
bladder volume is very small. The irritation may
result from urinary bladder infection or
tumors, increased acidity of the urine, or detrusor
hyper-reflexia.
• Incontinence, an inability
to control urination voluntarily,
may involve periodic involuntary urination, or a continual, slow trickle of urine from the urethra. Incontinence may
result from urinary bladder or urethral
problems, damage or weakening of the muscles of the
pelvic floor, or interference with normal sensory
or motor innervation in the region. Renal
function and daily urinary volume are normal.
• Changes in the volume of urine
produced indicate that there are problems either at
the kidneys or with the control of renal
function.
Normal daily urine output depends on the age:
-
1 month – 300 ml;
-
6 month – 400 ml;
-
1 year – 600 ml;
-
1-10 years – we have the formula to calculate daily
urine output:
V
= 600 + 100 (n-1), n – age of the patient;
-
older then 10 years – 1500 ml.
Volume of single urination:
-
0-6 months – 30 ml;
-
7-12 months – 60 ml:
-
5 years – 100 ml;
-
primary school age – 150 ml;
-
senior school age – 250 ml.
Polyuria, the production of excessive amounts of urine (2 times more than normal for
age), may result from
hormonal or metabolic problems,
such as those associated with diabetes, or damage to the glomeruli, as in glomerulonephritis.
Oliguria (daily amount of urine is less than
1/4 normal age volume)
and anuria (decrease
in urine up to 5% of daily volume and a complete cessation of urination during
the day) are
conditions that indicate serious kidney problems and potential renal failure. Renal failure can occur with heart
failure, renal ischemia, circulatory shock, burns, and a variety of
other disorders.
Dysuria (painful or difficult urination)
may occur with cystitis and urinary
obstructions.
Urinary frequency: Is an abnormally frequent
voiding. It is expressed in terms of day to night ratio. It results from polyuria or from a decrease in the
functional bladder capacity
as in bladder irritation or inflammation
Nocturia: Implies the need to rise during
hours of sleep to empty the bladder.
Dysuria: Is a specific form of discomfort
arising from the urinary tract in which there is pain immediately before, during or
immediately after micturation
Urgency: Is the loss of the normal ability
to postpone micturation beyond the time when the desire to pass urine is initially perceived
Incontinence: Refers to an involuntary loss of
urine that has become a social or hygienic problem
Hesitancy: Is difficulty initiating the
process of micturation.
Terminal dribbling: is difficulty of completing
micturation in a clean stop fashion.
Important clinical signs of urinary
system disorders include the following:
• Hematuria, the
presence of red blood cells in the urine, indicates bleeding at the kidneys or conducting system. Hematuria
producing dark red urine usually indicates
bleeding in the kidney, and
hematuria producing bright red urine indicates bleeding in the lower urinary tract.
Hematuria most commonly occurs with
trauma to the kidneys, calculi (kidney
stones), tumors, or urinary tract infections.
• Hemoglobinuria is
the presence of hemoglobin in the urine. Hemoglobinuria indicates increased hemolysis of red blood
cells within the circulation, due to
cardiovascular or metabolic problems. Examples of conditions resulting in hemoglobinuria include the thalassemias,
sickle cell anemia, hypersplenism, and some autoimmune disorders.
• Changes in urine color may accompany some renal disorders. For example, the
urine may become (1) cloudy, due to the
presence of bacteria, lipids,
or epithelial cells; (2) red or brown from hemoglobin or myoglobin; (3) blue-green from bilirubin; or (4) brown-black
from excessive concentration. Not all color
changes are abnormal, however. Some foods and
several prescription drugs can cause
changes in urine color. A serving of beets can give
urine a reddish color, whereas eating rhubarb can
give urine an orange tint, and B
vitamins turn it a vivid
yellow.
• Renal disorders typically lead to
protein loss in the urine (proteinuria) and if
severe, results in a generalized
edema in peripheral tissues. Facial swelling, especially around the eyes, is often seen.
Secondary
signs of renal diseases
• Chills,
• Headache,
• Dizziness,
• Visual disorders,
• Heart pain,
• Skin itching,
• Loss of appetite,
• Nausea,
• Vomiting ,
• Fever.
A fever
commonly develops when the urinary system is infected by pathogens. Urinary bladder infections (cystitis) often
result in a lowgrade fever;
kidney infections, such as pyelonephritis, usually produce very high fevers.
Renal edema
Oedema of
renal aetiology is quite specific in most cases and can easily be
differentiated from oedema of other origin, e.g. cardiac oedema, by the
affection of loose connective tissue (the eyelids, the face) rather than of the
lower extremities. Renal oedema can develop and resolve quickly. In pro nounced
cases, oedema is usually uniform over the entire trunk and the ex tremities
(anasarca). Not only the skin but also subcutaneous fat and the internal organs
become oedematous. The liver usually becomes oedematous and enlarged, but in
renal diseases the enlargement of the liver is usually proportional to
enlargement of the other ograns, and is never so pronounced as in cardiac
oedema. Greater or lesser amount of fluid is ac cumulated in the serous
cavities, e.g. in the pleural, abdominal, and pericardial cavities. Oedema can
be revealed by palpation. It can also be confirmed by the McClure-Aldrich test:
0.2 ml of isotonic sodium chloride solution is injected into the skin on the
median surface of a forearm and the time of disappearance of the resulting weal
is noted. In a healthy sub ject, the weal is resolved within one hour. In the
presence of a marked oedematous syndrome, the dynamics of oedema during
treatment can be better assessed by repeating the test in several days with
measurement of girths of the extremities and the abdomen at the same level, by
determining the fluid level in the pleural and abdominal cavities, by weighing
the pa tient, and also by determining daily diuresis and water balance of the
body (the ratio of the taken and eliminated liquid during 24-hour period).
Oedema, like the general
disorder in the water-salt metabolism, arises due to various causes in renal
diseases.
1. Diffuse increased
permeability of the capillary wall is important in development of
oedema in many diseases of the kidneys attended by the oedematous syndrome.
Great importance in this process is attributed to auto-immune processes and
increased hyaluronidase activity of the blood serum, which as a rule, attends many diseases of the kidneys
Hyaluronidase intensifies
depolymerization of hyaluronic complexes of mucopolysaccharides that form
the intercellular substance (interen-dothelial "cement") and the
basal membrane of the capillary wall. Porosity of the wall thus increases. The
decreased blood serum content of calcium is also important because calcium
compounds with protein (calcium pro-teinate) is a component part of the
intercellular "cement"; change in the blood pH (acidosis) is important
as well. Because of the generalized increase in capillary permeability, not
only water and the dissolved substances, but also much protein pass from
the blood to the tissues. Depolymerization of mucopolysaccharides of the
intercellular substance of tissues increases the quantity of molecules in
the intercellular fluid and raises its colloidal-osmotic pressure.
It follows that the nephrotic
syndrome is characterized not only by in creased permeability of the capillary
wall that facilitates fluid transport to the tissues, but also conditions
are provided for fluid retention in the tissues, because the increased
colloidal-osmotic pressure of the intercellular fluid accounts for its
hydrophilic property: the intercellular fluid easier ab sorbs water and gives
it back with difficulty. The comparatively high protein content in the oedema
fluid (transudate) explains the higher density and lower mobility of
oedema in the presence of deranged capillary permeability compared with oedema
associated with hypoproteinaemia.
In the presence of increased
capillary permeability, transudate is ac cumulated in the subcutaneous fat and
other highly vascularized tissues. Serous cavities usually contain low amounts
of fluid. Disordered capillary permeability in the glomeruli causes
proteinuria and promotes develop-i ment of hypoproteinaemia. Oedema of this
type occurs not only in diseases of the kidneys but in some other diseases
as well, e.g. it can also be allergic or angioneurotic (Quincke's oedema),
in cases with bee stinging, etc.
2. Colloidal-osmotic
(hypoproteinaemic) mechanism of oedema development is also important
in the nephrotic syndrome. It is manifested in a decreased plasma oncotic
pressure due to high proteinuria which usually occurs in such patients, and
also in protein passage through the porous capillary walls into the
tissues. Oedema of predominantly colloidal-osmotic origin obeys the laws of
hydrostatics and tends to develop in the first instance in the lower
extremities in walking patients and in the loin of bed-ridden patients.
Hypoproteinaemic oedema usually occurs in cases where the blood protein
content is less than 35-40 g/1 (3.5-4 g/100 ml) and albumins are contained in
the quantity below 10-15 g^/1 (1-1.5 g/100 ml). Qualitative changes in the composition
of the blood proteins are very important. Highly dispersed proteins (albumins)
are mainly lost in the urine in nephritis patients; the amount of
globulins decreases to a lesser extent. Osmotic pressure is determined by
the quantity of molecules contained in a unit volume of blood plasma
rather t their molecular weight. The loss of highly dispersed albumins,
specific colloidal-osmotic pressure is about three times that
of dispersion globulins, therefore substantially decreases oncotic
pres the blood.
Hypoproteinaemic oedema arises
not only in the nephrotic syndr can also develop in long starvation
(hunger oedema), deranged abs( in the small intestine (disordered absorption
syndrome), cancer cai and in some other diseases attended by a decreased protein
contenl blood plasma.
3. Hypernatriaemic
oedema (to be more exact, hypernatr: oedema) is explained by the
retention of the highly hydrophilic sodiu in 4he blood and especially in
the tissues. Administration of I chloride in large doses can thus cause
this oedema. Hypernatriahi tending diseases of the kidneys is an additional
factor intensifying feet of increased capillary permeability and
hypoproteinaemia. He factors, and in the first instance hypersecretion of
aldosterone (the s cortex hormone) and antidiuretic hormone (the posterior
pituitai mone), are very important in the accumulation of the sodium ion
i diseases.
Any oedema, irrespective of
its intensity, indicates upset osmo tion in which the hormone link
(aldosterone-antidiuretic hormone s is the decisive one. This hormone
system is mainly responsible for taining constant volume and ionic
composition of the blood, volume of circulating blood decreases even
insignificantly (which cai in renal diseases when part of the liquid
passes from the blood to due to increased porosity of the capillary wall
or decreased oncotic p of the blood), the volume receptors, located mainly
in the walls of tr atrium and the common carotids, are stimulated.
Protective mech respond to this stimulation to maintain the intravascular
v Aldosterone secretion by the adrenal cortex is intensified to ii sodium
reabsorption in the walls of the renal tubules and its concen in the
blood, and to promote its accumulation in tissues. Accorc some authors,
the quantity of aldosterone excreted in the urine dui hours increases in
the nephrotic oedema from 2-10 to 25-200/ more. Sodium excretion in the
urine thereby decreases considi Secondary hypersecretion of aldosterone
that develops as a comper reaction, e.g. in oedema or a sudden loss of water from
the body, is secondary hyperaldosteronism as distinct from the
phyperaldosteronism that occurs in tumours or hypertrophy of the a cortex.
Increased sodium reabsorption in the renal tubules is follo\ increased
reabsorption .of water. High concentration of the
sodium lood (due to its intensified reabsorption in the renal
tubules) lates osmoreceptors and intensifies secretion of the antidiuretic
hor by the pituitary gland, which in turn intensifies the
facultative reab-ion of water in distal tubules still more. If the primary
cause of na (increased capillary permeability, decreased oncotic pressure of
ixa) is still active, fluid is not retained in the blood vessels and con-s its
passage from the blood to the tissues to intensify oedema. . Oedema can
occur in acute anuria of the kidneys in acute
poisoning with corrosive sublimate), hypovolaemic reduction of blood
circula-in the kidneys (profuse blood loss, shock), and also in the
terminal e of certain chronic renal diseases (retention oedema). But
decreased aerular filtration becomes only important in the presence of
other runners of oedema rather than an independent factor. For example,
in ire renal insufficiency attended by pronounced filtration
disturbances, ema is often absent or even resolved, if any.
It should also be noted that
none of the above mechanisms of renal ema develops independently but
becomes only a dominating factor in i or that case.
Renal hypertension
Renal arterial hypertension is a
symptomatic hypertension caused by the affection of the kidneys or renal
vessels and upset renal mechanism of arterial pressure regulation. Among all
cases of arterial hypertension, renal hypertension makes about 10—15 per cent.
Many diseases of the kidneys, in
the first instance acute and chronic glomerulonephritis, pyelonephritis,
nephrosclerosis and various affections of the renal blood vessels are attended
by elevated arterial pressure. This is underlined by the important role that
the kidneys play in the regulation of arterial pressure. The juxtaglomerular
apparatus of the kidneys, which is an accumulation of special cells at the
vascular pole of the glomerulus, the point where the artery nears the proximal
end of the distal convoluted tubule, produces renin in the presence of
ischaemia of the renal parenchyma. Renin acts on the liver-produced
hypertensinogen, which is the conversion of a2-globulin of plasma,
to convert it into angiotensinogen. This converted enzymatically into
angiotensin (hypertensin).
At later stages, dystrophic changes
occur in the myocardium because vascularization lags behind the growth of the
muscle weight to account for the deficient blood supply; next, cardiosclerosis
develops. At the time, atherosclerosis of the coronary vessels may develop due
to upsemetabolism, which is characteristic for arterial hypertension and other
renal diseases attended by the nephrotic syndrome. The coi disease impairs
blood supply to the myocardium to an even greater pain, like that of angina
pectoris often occurs. Further progress of diseases can provoke circulatory
insufficiency, urtain acute diseases of the kidneys attended by a rapid and
pronounc-:vation of the arterial pressure, mainly acute glomerulonephritis, are
Jed by the condition at which the left ventricle is not hypertrophied >h to
compensate for the markedly increased load. Acute ventricular e can therefore
develop. It is manifested by attacks of cardiac asthma ven by a lung oedema.
It follows therefore that in certain kidney diseases,
the renal hypertension syndrome can be of primary significance in the clinical
picture of the disease and can be decisive for its course and outcome.
Physical examination
Examination
swelling: on the limbs, face,
sacral region, lower abdomen, absent. Muscle tremor, noisy breathing,
hemorrhages on the skin, nasal bleeding, smell of urine and ammonium from the
mouth, signs are not found. Lumbar region: prominence, redness, light swelling,
absent.


Image
3. Edema (swelling) of feet.
Kidney
palpation in vertical and horizontal
position: are not palpated. Shape, size, consistency, mobility, level of ptosis
(palpated kidney, mobile kidney, “migrating” kidney). Surface, painfulness.
Palpation of the left kidney is done first, which
is normally impalpable. With the right hand placed
anteriorly in the left lumbar region and the left one posteriorly in the left
loin, the patient is asked to take a deep breath in. If the kidney
is enlarged a firm swelling will be felt
between the two hands. (I.e. bimanually palpable). The right
kidney can be felt in much the same way as the left. The lower pole of the
right kidney, unlike the left, is commonly palpable in thin
patients.
The urinary bladder is not palpable normally. When it is full, a smooth, firm,
regular oval shaped swelling will be palpated in the suprapubic region
and its upper border may reach as far as the umbilicus. The lateral and upper borders
can be readily made out, but it is not possible to feel its lower border (i.e. the swelling
arises out of the pelvis). It is dull to
percussion. A full bladder will have sided to side
mobility but not up and down
Percussion
of renal region: Pasternatskiy’s symptom: positive, in the right, in
the left, on both sides, painfulness during urination, negative.
Frequency of urination in the day, day or night
non-keeping of the urine, painfulness during urination, no changes.
In suspected
urinary tract disorders, further assessment by laboratory, radiologic, and other evaluative
methods is carried out.
Complex of laboratory investigation:
1.
Urineanalysis once per 7-10 days.
2.
Nechiporenco (Amburgeau,Kakovskiy-Addis) test.
3. Revealing
of the so-called “active leukocytes” in the urine sediment has some auxiliary
significance.
4. Urine
inoculation (not less than 3 times) with definition of microbe sensitivity to
antibiotics.
5.
Determination of bacteriuria degree. It is considered significant if there are
100000 of microbes in 1 ml of urine.
6.
Determination of renal function condition with Zimnitsky’s test (takes 8 urine
portion once per 3 hours)
7. Rebergs
test
8.
Determination of secretory renal function and renal blood flow. Function of
distal nephrons (ammonia, filtrated acidity of urine), proximal tubules
(α2-microglobulin in urine, proteinuria, calciuria, phosphaturia), Henle’s
loop (osmotic concentration of the urine).
9. Biochemical
analyses of blood: total protein, cholesterole, residual nitrogen, creatine,
blood urea, dysproteinemia (with elevated levels of α-and
γ-globulins), rise of ciliac acids, mucoproteis, positive C-reactive
protein reaction.
10. Ultrasonography of kidneys
and urinary bladder.
11. Urography, excretory
urography, cystography and cyctoscopy.
General analyses of the urine:
Collect the morning urine, middle portion;
inverstigate physical properties, and lead microscopy.
Urine physical properties:
·
clearness,
pH, specific gravity,
·
methods
chemical properties: protein, glucose, sugar, ketone bodies, biliary pigments
·
microscopy
of sediment: leukocytes, erythrocytes, cylinders, endotelial cells
Common rules of urine collection:
The first portion of urine have to be taking after
slipping in the morning. Before taking the analysis the patient must be washed
and he have to collect the urine in the clear bottle, then send it to laboratory.
Bacteriological investigation: 10 ml of urine in the sterile test-tube.
Urinalysis can reveal diseases that have gone unnoticed
because they do not produce striking signs or symptoms. Examples include
diabetes mellitus, various forms of glomerulonephritis, and chronic urinary
tract infections.
The most cost-effective device used to screen urine is a
paper or plastic dipstick. This microchemistry system has been available for
many years and allows qualitative and semi-quantitative analysis within one minute
by simple but careful observation. The color change occurring on each segment
of the strip is compared to a color chart to obtain results. However, a
careless doctor, nurse, or assistant is entirely capable of misreading or
misinterpreting the results. Microscopic urinalysis requires only a relatively
inexpensive light microscope.
MACROSCOPIC URINALYSIS
The first part of a urinalysis is direct visual
observation. Normal, fresh urine is pale to dark yellow or amber in color and
clear. Normal urine volume is 750 to 2000 ml/24hr.
Turbidity or cloudiness may be caused by excessive
cellular material or protein in the urine or may develop from crystallization
or precipitation of salts upon standing at room temperature or in the
refrigerator. Clearing of the specimen after addition of a small amount of acid
indicates that precipitation of salts is the probable cause of tubidity.
A red or red-brown (abnormal) color could be from a food
dye, eating fresh beets, a drug, or the presence of either hemoglobin or myoglobin.
If the sample contained many red blood cells, it would be cloudy as well as
red.

Image
4. Three urine samples are shown. The one
at the left shows a red, cloudy appearance. The one in the center is red but
clear. The one on the right is yellow, but cloudy.
URINE DIPSTICK CHEMICAL ANALYSIS
pH
The glomerular filtrate of blood plasma is usually
acidified by renal tubules and collecting ducts from a pH of 7.4 to about
Specific gravity (sp gr)
Specific gravity (which is directly proportional to urine
osmolality which measures solute concentration) measures urine density, or the
ability of the kidney to concentrate or dilute the urine over that of plasma.
Dipsticks are available that also measure specific gravity in approximations.
Most laboratories measure specific gravity with a refractometer.
Specific gravity between 1.002 and 1.035 on a random
sample should be considered normal if kidney function is normal. Since the sp
gr of the glomerular filtrate in Bowman's space ranges from 1.007 to 1.010, any
measurement below this range indicates hydration and any measurement above it
indicates relative dehydration.
Relative density of urine (specific weight) normally
in common analysis is 1,017-1,024 (daily fluctuations are 1,004-1,040), it
reflects concentrational and excretoric function of
kidneys. Changes of relative density of urine are called hypostenuria
(decreasing), hyperstenuria (increasing), isostenuria (monotonous).
Hypoisostenuria is a sign of decreasing of functional
ability of kidneys.
If sp gr is not > 1.022 after a 12 hour period without
food or water, renal concentrating ability is impaired and the patient either
has generalized renal impairment or nephrogenic diabetes insipidus. In
end-stage renal disease, sp gr tends to become 1.007 to 1.010.
Any urine having a specific gravity over 1.035 is either
contaminated, contains very high levels of glucose, or the patient may have
recently received high density radiopaque dyes intravenously for radiographic
studies or low molecular weight dextran solutions. Subtract 0.004 for every 1%
glucose to determine non-glucose solute concentration.
Protein
Dipstick screening for protein is done on whole urine,
but semi-quantitative tests for urine protein should be performed on the
supernatant of centrifuged urine since the cells suspended in normal urine can
produce a falsely high estimation of protein. Normally, only small plasma
proteins filtered at the glomerulus are reabsorbed by the renal tubule. However,
a small amount of filtered plasma proteins and protein secreted by the nephron
(Tamm-Horsfall protein) can be found in normal urine. Normal total protein
excretion does not usually exceed 150 mg/24 hours or 10 mg/100 ml in any single
specimen. More than 150 mg/day is defined as proteinuria. Proteinuria > 3.5
gm/24 hours is severe and known as nephrotic syndrome.
Dipsticks detect protein by production of color with an
indicator dye, Bromphenol blue, which is most sensitive to albumin but detects
globulins and Bence-Jones protein poorly. Precipitation by heat is a better
semiquantitative method, but overall, it is not a highly sensitive test. The
sulfosalicylic acid test is a more sensitive precipitation test. It can detect
albumin, globulins, and Bence-Jones protein at low concentrations.
In rough terms, trace positive results (which represent a
slightly hazy appearance in urine) are equivalent to 10 mg/100 ml or about 150
mg/24 hours (the upper limit of normal). 1+ corresponds to about 200-500 mg/24
hours, a 2+ to 0.5-1.5 gm/24 hours, a 3+ to 2-5 gm/24 hours, and a 4+
represents 7 gm/24 hours or greater.
Glucose
Less than 0.1% of glucose normally filtered by the
glomerulus appears in urine (< 130 mg/24 hr). Glycosuria (excess sugar in urine)
generally means diabetes mellitus. Dipsticks employing the glucose oxidase
reaction for screening are specific for glucos glucose but can miss other
reducing sugars such as galactose and fructose. For this reason, most newborn
and infant urines are routinely screened for reducing sugars by methods other
than glucose oxidase (such as the Clinitest, a modified Benedict's copper
reduction test).
Ketones
Ketones (acetone, aceotacetic acid, beta-hydroxybutyric
acid) resulting from either diabetic ketosis or some other form of calorie
deprivation (starvation), are easily detected using either dipsticks or test
tablets containing sodium nitroprusside.
Nitrite
A positive nitrite test indicates that bacteria may be
present in significant numbers in urine. Gram negative rods such as E. coli are
more likely to give a positive test.
Leukocyte Esterase
A positive leukocyte esterase test results from the
presence of white blood cells either as whole cells or as lysed cells. Pyuria
can be detected even if the urine sample contains damaged or lysed WBC's. A
negative leukocyte esterase test means that an infection is unlikely and that,
without additional evidence of urinary tract infection, microscopic exam and/or
urine culture need not be done to rule out significant bacteriuria.
MICROSCOPIC URINALYSIS
Methodology
A sample of well-mixed urine (usually 10-15 ml) is
centrifuged in a test tube at relatively low speed (about 2-3,000 rpm) for 5-10
minutes until a moderately cohesive button is produced at the bottom of the
tube. The supernate is decanted and a volume of 0.2 to 0.5 ml is left inside
the tube. The sediment is resuspended in the remaining supernate by flicking
the bottom of the tube several times. A drop of resuspended sediment is poured
onto a glass slide and coverslipped.
Examination
The sediment is first examined under low power to
identify most crystals, casts, squamous cells, and other large objects. The
numbers of casts seen are usually reported as number of each type found per low
power field (LPF). Example: 5-10 hyaline casts/L casts/LPF. Since the number of
elements found in each field may vary considerably from one field to another,
several fields are averaged. Next, examination is carried out at high power to
identify crystals, cells, and bacteria. The various types of cells are usually
described as the number of each type found per average high power field (HPF).
Example: 1-5 WBC/HPF.
Hematuria is the presence of abnormal numbers of red
cells in urine due to: glomerular damage, tumors which erode the urinary tract
anywhere along its length, kidney trauma, urinary tract stones, renal infarcts,
acute tubular necrosis, upper and lower uri urinary tract infections,
nephrotoxins, and physical stress. Red cells may also contaminate the urine
from the vagina in menstruating women or from trauma produced by bladder
catherization. Theoretically, no red cells should be found, but some find their
way into the urine even in very healthy individuals. However, if one or more
red cells can be found in every high power field, and if contamination can be
ruled out, the specimen is probably abnormal.
RBC's may appear normally shaped, swollen by dilute urine
(in fact, only cell ghosts and free hemoglobin may remain), or crenated by
concentrated urine. Both swollen, partly hemolyzed RBC's and crenated RBC's are
sometimes difficult to distinguish from WBC's in the urine. In addition, red
cell ghosts may simulate yeast. The presence of dysmorphic RBC's in urine
suggests a glomerular disease such as a glomerulonephritis. Dysmorphic RBC's
have odd shapes as a consequence of being distorted via passage through the
abnormal glomerular structure.

Image
5. The presence of this red blood cell
cast in on urine microscopic analysis suggests a glomerular or renal tubular
injury.
White Blood Cells
Pyuria refers to the presence of abnormal numbers of
leukocytes that may appear with infection in either the upper or lower urinary
tract or with acute glomerulonephritis. Usually, the WBC's are granulocytes. White
cells from the vagina, especially in the presence of vaginal and cervical
infections, or the external urethral meatus in men and women may contaminate
the urine.
If two or more leukocytes per each high power field
appear in non-contaminated urine, the specimen is probably abnormal. Leukocytes
have lobed nuclei and granular cytoplasm.
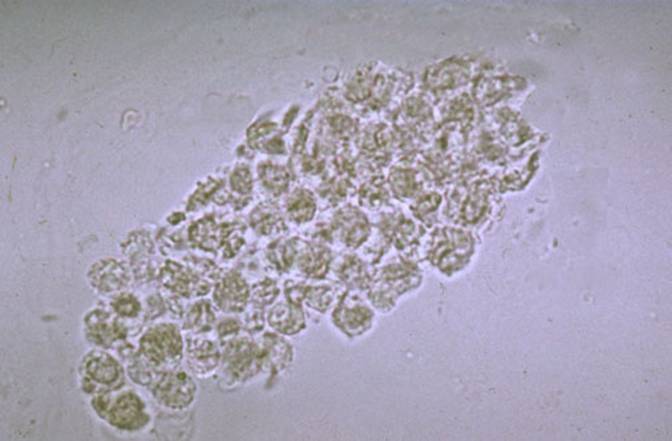
Image
6. This white blood cell cast suggests an acute
pyelonephritis
Epithelial Cells
Renal tubular epithelial cells, usually larger than
granulocytes, contain a large round or oval nucleus and normally slough into
the urine in small numbers. However, with nephrotic syndrome and in conditions
leading to tubular degeneration, the number sloughed is increased.
When lipiduria occurs, these cells contain endogenous
fats. When filled with numerous fat droplets, such cells are called oval fat
bodies. Oval fat bodies exhibit a "Maltese cross" configuration by
polarized light microscopy.
Transitional epithelial cells from the renal pelvis,
ureter, or bladder have more regular cell borders, larger nuclei, and smaller
overall size than squamous epithelium. Renal tubular epithelial cells are
smaller and rounder than transitional epithelium, and their nucleus occupies
more of the total cell volume.
Squamous epithelial cells from the skin surface or from
the outer urethra can appear in urine.
Their significance is that they represent possible
contamination of the specimen with skin flora.

Image
7. Large polygonal squamous epithelial cells with
small nuclei are seen here.
Urinary casts are formed only in the distal convoluted
tubule (DCT) or the collecting duct (distal nephron). The proximal convoluted
tubule (PCT) and loop of Henle are not locations for cast formation. Hyaline
casts are composed primarily of a mucoprotein (Tamm-Horsfall protein) secreted
by tubule cells. The Tamm-Horsfall protein secretion (green dots) is
illustrated in the diagram below, forming a hyaline cast in the collecting
duct:
Even with glomerular injury causing increased glomerular
permeability to plasma proteins with resulting proteinuria, most matrix or
"glue" that cements urinary casts together is Tamm-Horsfall
mucoprotein, although albumin and some globulins are also incorporated. An
example of glomerular inflammation with leakage of RBC's to produce a red blood
cell cast is shown in the diagram below:
The factors which favor protein cast formation are low
flow rate, high salt concentration, and low pH, all of which favor protein
denaturation and precipitation, particularly that of the Tamm-Horsfall protein.
Protein casts with long, thin tails formed at the junction of Henle's loop and
the distal convoluted tubule are called cylindroids. Hyaline casts can be seen
even in healthy patients.

Image 8. Hyaline casts, which appear very pale and slightly refractile, are common
findings in urine
Red blood cells may stick together and form red blood
cell casts. Such casts are indicative of glomerulonephritis, with leakage of
RBC's from glomeruli, or severe tubular damage.
White blood cell casts are most typical for acute
pyelonephritis, but they may also be present with glomerulonephritis. Their
presence indicates inflammation of the kidney, because such casts will not form
except in the kidney.
When cellular casts remain in the nephron for some time
before they are flushed into the bladder urine, the cells may degenerate to
become a coarsely granular cast, later a finely granular cast, and ultimately,
a waxy cast. Granular and waxy casts are be believed to derive from renal
tubular cell casts. Broad casts are believed to emanate from damaged and
dilated tubules and are therefore seen in end-stage chronic renal disease.
The so-called telescoped urinary sediment is one in which
red cells, white cells, oval fat bodies, and all types of casts are found in
more or less equal profusion. The conditions which may lead to a telescoped
sediment are: 1) lupus nephritis 2) malignant hypertension 3) diabetic
glomerulosclerosis, and 4) rapidly progressive glomerulonephritis.
In end-stage kidney disease of any cause, the urinary
sediment often becomes very scant because few remaining nephrons produce dilute
urine.

Image
9. This renal tubular cell cast suggests
injury to the tubular epithelium

Image
10. These are
granular casts, with a roughly rectangular shape.
Bacteria
Bacteria are common in urine specimens because of the
abundant normal microbial flora of the vagina or external urethral meatus and
because of their ability to rapidly multiply in urine standing at room
temperature. Therefore, microbial organisms found in all but the most
scrupulously collected urines should be interpreted in view of clinical
symptoms.
Diagnosis of bacteriuria in a case of suspected urinary
tract infection requires culture. A colony count may also be done to see if
significant numbers of bacteria are present. Generally, more than 100,000/ml of
one organism reflects significant bacteriuria. Multiple organisms reflect
contamination. However, the presence of any organism in catheterized or suprapubic
tap specimens should be considered significant.
Yeast
Yeast cells may be contaminants or represent a true yeast
infection. They are often difficult to distinguish from red cells and amorphous
crystals but are distinguished by their tendency to bud. Most often they are
Candida, which may colonize bladder, urethra, or vagina.
Common crystals seen even in healthy patients include
calcium oxalate, triple phosphate crystals and amorphous phosphates.

Image 11.

Image 12. These are
oxalate crystals, which look like little envelopes (or tetrahedrons, depending
upon your point of view). Oxalate crystals are common.

Image
13. These "triple phosphate"
crystals look like rectangles, or coffin lids if you are feeling depressed

Image
14. These cystine crystals are shaped like stop signs.
Cystine crystals are quite rare
Very uncommon crystals include: cystine crystals in urine
of neonates with congenital cystinuria or severe liver disease, tyrosine
crystals with congenital tyrosinosis or marked liver impairment, or leucine
crystals in patients with severe liver disease or with maple syrup urine
disease.
METHODS OF URINE COLLECTION
1.
Random
collection taken at any time of day with no precautions regarding
contamination. The sample may be dilute, isotonic, or hypertonic and may
contain white cells, bacteria, and squamous epithelium as contaminants. In
females, the specimen may cont contain vaginal contaminants such as
trichomonads, yeast, and during menses, red cells.
2.
Early
morning collection of the sample before ingestion of any fluid. This is usually
hypertonic and reflects the ability of the kidney to concentrate urine during
dehydration which occurs overnight. If all fluid ingestion has been avoided
since 6 p.m. the previous day, the specific gravity usually exceeds
3.
Clean-catch,
midstream urine specimen collected after cleansing the external urethral
meatus. A cotton sponge soaked with benzalkonium hydrochloride is useful and
non-irritating for this purpose. A midstream urine is one in which the first
half of the bladder urine is discarded and the collection vessel is introduced
into the urinary stream to catch the last half. The first half of the stream
serves to flush contaminating cells and microbes from the outer urethra prior
to collection. This sounds easy, but it isn't (try it yourself before
criticizing the patient).
4.
Catherization
of the bladder through the urethra for urine collection is carried out only in
special circumstances, i.e., in a comatose or confused patient. This procedure
risks introducing infection and traumatizing the urethra and bladder, thus
producing iatrogenic infection or hematuria.
5.
Suprapubic
transabdominal needle aspiration of the bladder. When done under ideal
conditions, this provides the purest sampling of bladder urine. This is a good
method for infants and small children.
|
|
Quantities methods:
Method
by Kakovsky-Addis:
In the clear bottle collect urine, which was excreted of
urine while 10 night’s hours (from 22 to 8). Count formed, elements of daily
urine:
Leucocytes/ erythrocytes as 2x10 6 /1x106
Ambyrze’s
method
Use for investigate “minute leukocyturia” formed
elements which excreted of urine while one minute leucocytes / erythrocytes as
2x10 6 / 1x106
Nechepurenko’s
method
Taking middle portion of urine, near 2-3 ml.
Count number formed elements in the 1 ml of urinary
sediment.
leucocytes /
erythrocytes as 2x10 6 /1x106
Zymnyckiy’s
test
Collect 8-portion urine while 24 hours; from 6 o’clock
(this portion do not take).While every 3 hours to the 6 of other day.
|
Test |
Leucocytes |
Erythrocytes |
Hyaline cylinders |
|
Amburge (in minute diuresis) |
To 2000-3000 |
To 1000 |
To 100 |
|
Nechipo-renko ( in 1 ml) |
To 4000 |
To 1000 |
To 220 |
|
Addis-Kakovsky (in daily diuresis) |
To 2 mln |
To 1mln |
To 2 thousands |
Biochemical examination of blood for estimation of function of kidneys
are: urea, creatinine, indican, RN , K +
, Na + , Mg++ and others.
Determination of klirens by creatinine allows estimating glomerular
filtration, renal plasma flow and other functions.
Measurement of glomerular filtration rate
The endogenous creatinine clearance (Ccr) in milliliters per minute
estimates the glomerular filtration rate (GFR). A 24-hour urine collection is
usually obtained; however, in small children from whom collection is difficult,
a 12-hour daytime specimen, collected when urine flow rate is greatest, is
acceptable. The procedure for collecting a timed urine specimen should be
explained carefully so that the parent or patient understands fully the
rationale of (1) first emptying the bladder (discarding that urine) and noting
the time; and (2) putting all urine subsequently voided into the collection
receptacle, including the last void, 12 or 24 hours later. Reliability of the
24-hour collection can be checked by measuring the total 24-hour creatinine
excretion in the specimen. Total daily creatinine excretion (creatinine index)
should be in the range of 14–20 mg/kg. Creatinine indices on either side of
this range suggest collections that were either inadequate or excessive.
Calculation by the following formula requires measurements of plasma creatinine
(Pcr) in mg/mL, urine creatinine (Ucr) in mg/mL, and
urine volume (V) expressed as mL/min:
Creatinine is a reflection of body
muscle mass. Because accepted ranges of normal Ccr are based on
adult parameters, correction for size is needed to determine normal ranges in
children. Clearance is corrected to a standard body surface area of
Although 80–125 mL/min/1.73 m2
is the normal range for Ccr, estimates at the lower end of this
range may indicate problems.
A simple and tested formula for quick approximation of Ccr incorporates
use of the plasma creatinine level and the child's length in centimeters:
Note: Because this formula takes into
account the body surface area, further correction is not necessary. Use 0.45 x
length in centimeters for newborns and for infants younger than age 1 year.
This method of calculation is not meant to detract from the importance of
clearance determinations, but is useful when a suspicious plasma creatinine
needs to be checked.
Additional instrumentary examinations are: X-ray examination -
excretory urography and ascending one with injection of iodine-containing
preparations such as urotrast, verographin and others; radioisotopic methods
such as renal scanning, isotopic renography; biopsy of kidneys; ultrasound
examination of kidneys.


Excretory urography
Renal
Ultrasound - Hydronephrosis


Renal
ultrasound
renal disease
UTI is a significant childhood
problem, probably second only to infection of the respiratory tract. Although
its exact incidence is not known, it is suggested that from 1% to 2% of
school-age children have UTI as demonstrated by significant bacteriuria. The
peak incidence of UTI not caused by structural anomalies occurs between 2 and 6 years
of age. Except for the neonatal period, females have a 10 to 30 times greater risk
for developing UTI than males. It has been estimated that approximately 5% of school-age females will develop
bacteriuria by 18 years of age. Such
statistics attest to the importance of preventing, diagnosing, and treating
this problem to prevent recurrent infections and possible renal damage in later
years.
Predisposing factors. A number of factors predispose
to the development of UTI. The major ones included here relate to anatomic,
physical, and chemical causes.
Anatomic and physical. These factors seem to account
for the increased incidence of bacteriuria in females. The short urethra, which
measures about
Introduction
of bacteria can occur in females during tub baths. Soap or water softeners
decrease the surface tension of the water, increasing the possibility of fluid
entry into the short urethra. Tight clothing or diapers, poor hygiene, and
local inflammation, such as from vaginitis or pinworm infestation, may also increase the risk of
ascending infection.
Physical
factors relating to the functioning of the bladder are of major importance in
the occurrence and spread of infection. Ordinarily urine is sterile, but at 37° C it is an excellent culture medium. Under
normal conditions the act of completely and repeatedly emptying the bladder
flushes away any organisms before they have an opportunity to multiply and
invade surrounding tissue. However, urine that remains in the bladder allows
bacteria from the urethra to rapidly become established in the rich medium.
Incomplete
bladder emptying may result from reflux, anatomic abnormalities, especially
involving the ureters, or dysfunction of the voiding mechanism. Vesicoureteral reflux (VUR)
refers to the retrograde flow of bladder urine into the ureters. Reflux increases
the chance for and perpetuates infection, since with each void urine is swept
up the ureters and then allowed to empty after voiding. Therefore, the residual
urine in the ureters remains in the bladder until the next void.
Primary reflux
results from the congenitally abnormal insertion of
the ureters into the bladder and predisposes to development of infection.
Secondary reflux occurs as a result of infection. Normally the ureters enter
the bladder wall in such a manner that the accumulating urine compresses the subrnucosal segment of the ureter, preventing reflux.
However, the edema caused by bladder infection
renders this mechanism at the ureterovesicular
junction incompetent. In addition, in infants and young children the shortness
of the subrnucosal portion of the ureter decreases the effectiveness of this
antireflux mechanism. Other causes of
secondary reflux are neurogenic bladder from either
chronic obstruction or neural dysfunction or as an iatrogenie result from progressive dilation of the ureters
following surgical urinary diversion.
Reflux with
infection can lead to kidney damage, since refluxed urine ascending into the
collecting tubules of the nephrons allows the
microorganisms to gain access to the renal parenchyma, initiating renal scarring.
Inflammation
of the kidney and upper tract (may be acute or chronic).
Acute or
chronic inflammatory disease resulting from infection may involve the kidneys
and upper urinary tract (pyelonephritis) or the bladder and lower tract
(cystitis).
Acute pyelonephritis
Onset of
disease based on the ground of acute bacterial and viral infections.
Diagnostic clinical criteria
1. Disuria -
frequent and painful micturitions (urination).
2. Painful
syndrome – lumbar region pains are present in the majority of school age
children.
3. The
temperature as a rule, febrile or subfebrile.
4. Urinary
syndrome consists of leucocyturia, normal or elevated diuresis, monotonous,
decreased specific gravity of the urine in different portions. Urine
inoculation - positive in 85% of cases.
5. Edematic
syndrome is absent.
6.
Hypertension is not typical.
7. Syndrome of
intoxication - weakness, indisposition, bad appetite, loss of weight, vomiting,
toxicosis, exicosis.
Main indices
of renal function are normal. Morphologic changes of kidneys are primary lesion
of interstitial renal tissue.
Glomerulonephritis
Glomerulonephritis
is an infectious allergic renal disease with primary lesions of glomerule.
Diagnostic clinical criteria
Clinical:
I.
Extrarenal symptoms:
1. Edema.
2. Arterial hypertension.
II.
Renal symptoms:
a) Oliguria and anuria are present in the initial period of acute
glomerulonephritis, in this case urine has high specific gravity (1030-1040 and
more),
b) hematuria of different degree - moderate
(microhematuria – when the quantity of RBC is less then 50) and massive
(macrohematuria - when the quantity of RBC is more then 50),
c) proteinuria:
·
moderate
- up to 1000 mg/l (daily loss is up to
·
significant
- more than 1000 mg/l. up to 2500-3000 mg/l (daily loss is 2,5-
- massive - more than 3000 mg/l (daily loss is more
than
d) leucocyturia - is not typical for
glomerulonephritis; may be transitory leucocyturia of lymphoid character,
e)
cylindruria - hyaline, epithelial, granular, waxy casts.
Nephrotic syndrome: massive
proteinuria, hypoproteinemia, hyperlipidemia, hypersholesterinemia, edemas.
Nephrytyc syndrome: hypertension, hematuria, moderate proteinuria, edemas.
Table 2
Prevention of urinary tract infection
|
Factors
|
|
|
Short female urethra close to
vagina and anus |
Perinea hygiene - wipe from
front to back. Avoid tub baths, especially with bubble
bath or water softener; use showers |
|
Avoid tight clothing or
diapers: wear cotton panties rather than nylon. Check for vaginitis or pinworms, especially
if child scratches between legs |
|
|
Incomplete emptying (reflux)
and overdis-tention of bladder |
Avoid “holding” urine;
encourage child to void frequently, especially before a long trip or other circumstances when
toilet facilities are not available |
|
Empty bladder completely with each void |
|
|
Avoid straining at stool |
|
|
Concentrated and alkaline urine |
Encourage generous fluid intake Acidify urine with juices such as apple
or cranberry and a diet high in animal protein |
Acute renal
failure (ARF)

ARF is an acute impairment of renal
function to exist when the kidneys suddenly are unable to regulate the volume
and composition of urine appropriately in response to food and fluid intake and
the needs of the organism.
Diagnostic
criteria: There are prerenal,
renal and postrenal (obstructive) ARF. The principal feature is oligoanuria associated with azotemia,
acidosis, and diverse electrolyte disturbances. ARF
is not common in childhood, but the outcome depends on the cause, associated
findings, and prompt recognition and treatment.
The terms “azotemia” and “uremia” are often used in relation to renal failure.
Azotemia is the accumulation of nitrogenous waste within the blood. Uremia is a
more advanced condition in which retention of nitrogenous products produces
toxic symptoms. Azotemia is not life threatening, whereas uremia is a serious
condition that often involves other body systems.
Important
causes of ARF:
I.
Prerenal:(decreased perfusion).
1. Acute gastroenteritis (vomiting, diarrhea, nasogastric tubes).
2. Acute anemia (hemolytic crises, including sickle cell
crisis).
3.
Shock.
4. Congestive heart failure
II.
Renal:
1. Acute tubular necrosis:
·
fluid
loss, hemorrhage, shock,
·
intravascular
hemolysis,
·
sepsis,
·
nephrotoxic
drugs, chemical, radiocontrast substances,
·
major
surgical procedures, road accidents, extensive burns,
·
hepatic
failure, congestive cardiac failure.
2. Glomerular disease:
·
acute
glomerulonephritis,
·
hemolitic
uremic syndrome.
3. Interstitial nephritis.
4. Acute bacterial pyelonephritis.
5. Miscellaneous:
·
snakebite,
·
renal
vein thrombosis.
III.
Post-renal (obstructive): Calculus, blood dots, crystals of uric acid,
sulphonamides.
Table 3
Laboratory findings associated with acute renal failure
|
Clinical problem |
Mechanism |
Clinical considerations |
|
Azotemia Elevated BUN levels |
Ongoing protein catabolism.
Significantly decreased excretion |
Lower rate of production in
neonates and persons with depleted protein stores. Increased in situations involving
large amounts of necrotic tissue or extravasated blood. |
|
Elevated plasma creatinine
levels |
Continued production.
Significantly decreased excretion |
Production less affected by other
factors. More sensitive measure of intensity of azotemia. Low in neonate
because of small muscle mass relative to size |
|
Metabolic acidosis |
Continued endogenous acid
production. Significantly decreased excretion. Depletion of extracellular and
intracellular fluid buffers. |
Compensatory hyperventilation.
Opisthotonos. Major threat to life. |
|
Hyponatremia |
Dilution of extracellular
fluid. Decreased excretion of water. |
May develop cerebral signs. |
|
Hyperkalemia |
Ongoing protein catabolism.
Decreased excretion compounded by metabolic acidosis. |
Most important electrolyte to
be considered in acute renal failure. May contribute to cardiac arrhythmia.
With ECG changes, major threat to life. Maybe lost from gastrointestinal
tract. |
|
Hypocatcemia |
Associated with metabolic
acidosis and hyper-phosphatemia. |
During alkali therapy, may
cause tetany. |
Chronic renal
failure (CRF)
The kidneys are
able to maintain the chemical composition of fluids within normal limits until
more than 50% of functional renal capacity is destroyed by disease or
injury. Chronic renal insufficiency or failure begins when the diseased kidneys
can no longer maintain normal chemical structure of body fluids under normal
conditions. Progressive deterioration over months or years produces a variety
of clinical and biochemical disturbances that eventually culminate in the
clinical syndrome known as uremia. The pattern of renal
dysfunction is remarkably uniform no matter what disease process initiates the
advanced disease. Renal vascular disorders such as hemolytic-uremic
syndrome, vascular thrombosis,
or cortical necrosis are less frequent causes.
Diagnostic criteria
I. Clinical:
· tiredness, fatigue, headache, loss of appetite,
vomiting,
· polyuria, nicturia, polydypsia, bone and joint pains,
retardation of growth, dryness and itching of skin,
· muscular convulsions, paresthesias, signs of sensor or
motor neuropathy,
· heart failure and hemodynamic disorders.
II. Laboratory:
· decrease of glomerular filtration rate,
· metabolic acidosis,
· anemia,
· decrease of thrombocytes’ adhesion,
· hyperkalemia, hyperphosphatemia, hypocalcemia,
hypoproteinemia, hyperuricemia,
· isostenuria,
· renal osteodystrophy,
· X-ray examination of the chest may reveal
cardiomegaly, hypertrophy of the left ventricle, aortectasia, lung’s edema,
pleural exudates.
Cause of chronic renal
failure
1.
Glomerular diseases.
a)
Glomerulonephritis:
-
of
unknown etiology,
-
associated
with systemic lupus erythematosus (SLE), polyarteriitis nodosa,
-
Henoch-Schonlein vasculitis.
b)
Familial nephropathy:
-
nephronophthisis,
-
Alport’s syndrome,
c)
Hemolytic uremic syndrome,
d)
Amyloidosis.
2.
Congenital anomalies:
a)
bilateral
renal dysplasia,
b)
congenital nephrotic syndrome,
c)
polycystic kidney.
Clinical manifestations
The first
evidence of difficulty is usually loss of normal energy and increased fatigue on
exertion. For example, the child may prefer quiet, passive activities rather
than participation in more active games and outdoor play. The child is usually
somewhat pale, but it is often so inconspicuous that the change may not be
evident to parents or others. Sometimes the blood pressure is elevated. As the
disease progresses, other manifestations may appear.
The child eats less well (especially breakfast), shows less interest in normal
activities, such as schoolwork or play, and has an
increased urinary output and a compensatory intake of fluid. For example, a
previously dry child may wet the bed at night. Pallor becomes more evident as
the skin develops a characteristic sallow, muddy appearance as the result of
anemia and deposition of urochrome pigment in the
skin. The child may complain of headache, muscle cramps, and nausea. Other
signs and symptoms include weight loss, facial puffiness,
malaise, bone or joint pain, growth retardation, dryness
or itching of the skin, bruised skin, and sometimes
sensory or motor loss. Amenorrhea is common in
adolescent girls.
The therapy is
generally instigated before the appearance of the uremic syndrome, although
there are occasions in which the symptoms may be
observed. Manifestations of untreated uremia
reflect the progressive nature of the homeostatic
disturbances and general toxicity. Gastrointestinal symptoms include anorexia and nausea
and vomiting. Bleeding tendencies are apparent in bruises, bloody diarrheal stools, stomatitis, and bleeding from lips
and mouth. There is intractable itching, probably related to hyperparathyroidism, and deposits of urea crystals
appear on the skin as “uremic frost”. There may be an unpleasant “uremic” odor
to the breath. Respirations become deeper as a result of metabolic acidosis, and circulatory overload is manifest by
hypertension, congestive heart failure, and
pulmonary edema. Neurologic involvement is
reflected by progressive confusion, dulling of sensorium,
and, ultimately, coma. Other signs may include tremors, muscular twitching, and seizures.
Oliguria
Background
Oliguria is defined as a urine
output that is less than 1 mL/kg/h in infants, less than 0.5 mL/kg/h in
children, and less than 400 mL daily in adults. It is one of the clinical
hallmarks of renal failure and has been used as a criterion for diagnosing and
staging acute kidney injury, previously referred to as acute renal failure. At
onset, oliguria is frequently acute. It is often the earliest sign of impaired
renal function and poses a diagnostic and management challenge to the
clinician.
Not all cases of acute kidney
injury are characterized by oliguria. Renal failure that results from
nephrotoxic injury, interstitial nephritis, or neonatal asphyxia is frequently
of the nonoliguric type, is related to a less severe renal injury, and has a
better prognosis. In addition, the degree of oliguria depends on hydration and
the concomitant use of diuretics.
In most clinical situations, acute
oliguria is reversible and does not result in intrinsic renal failure. However,
identification and timely treatment of reversible causes is crucial because the
therapeutic window may be small.
Patient education
For patient education information,
see the Diabetes Center, as well as Acute Kidney Failure and Chronic Kidney
Disease.
Etiology
Oliguria may result from prerenal,
intrinsic renal, or postrenal processes.
Prerenal failure
Prerenal insufficiency is a
functional response of structurally normal kidneys to hypoperfusion. Globally,
prerenal insufficiency accounts for approximately 70% of community-acquired
cases of acute renal failure and as many as 60% of hospital-acquired cases. A
decrease in circulatory volume evokes a systemic response aimed at normalizing
intravascular volume at the expense of the glomerular filtration rate (GFR).
Pathogenesis of prerenal failure.
Baroreceptor-mediated activation of
the sympathetic nervous system and renin-angiotensin axis results in renal
vasoconstriction and the resultant reduction in the GFR.
The early phase
of renal compensation for reduced perfusion includes autoregulatory maintenance
of the GFR via afferent arteriolar dilatation (induced by myogenic responses,
tubuloglomerular feedback, and prostaglandins) and via efferent arteriolar
constriction (mediated by angiotensin II). These changes are shown in the image
below.
Compensatory mechanisms for
preventing a fall in glomerular filtration rate (GFR) in the presence of
prerenal failure.
The early phase also includes
enhanced tubular reabsorption of salt and water (stimulated by the renin-angiotensin-aldosterone
system and sympathetic nervous system). Rapid reversibility of oliguria
following timely reestablishment of renal perfusion is an important
characteristic and is the usual scenario in prerenal insufficiency. For
example, oliguria in infants and children is most often secondary to
dehydration and reverses without renal injury if the dehydration is corrected.
However, prolonged renal hypoperfusion can result in a deleterious shift from
compensation to decompensation.
This decompensation phase is
characterized by excessive stimulation of the sympathetic and renin-angiotensin
systems, with resultant profound renal vasoconstriction and ischemic renal
injury.
Iatrogenic interference with renal
autoregulation by administration of vasoconstrictors (eg, cyclosporine,
tacrolimus), inhibitors of prostaglandin synthesis (eg, nonsteroidal
anti-inflammatory drugs), or angiotensin-converting enzyme (ACE) inhibitors can
precipitate oliguric acute renal failure in individuals with reduced renal perfusion.
Intrinsic renal failure
Intrinsic renal failure is
associated with structural renal damage. This includes acute tubular necrosis
(from prolonged ischemia, drugs, or toxins), primary glomerular diseases, or
vascular lesions.
Advancements in the care of
critically ill neonates, infants with congenital heart disease, and children
who undergo bone marrow and solid organ transplantation have led to a dramatic
broadening of the etiology of pediatric acute kidney injury. Although
multicenter etiologic data on pediatric acute renal failure are not available,
single-center data and literature reviews from the 1980s and 1990s reported
hemolytic uremic syndrome and other primary renal diseases as the most
prevalent causes.
Subsequent single-center data have
detailed the underlying causes of pediatric acute renal failure in large
cohorts of children. In a study of 226 children with acute renal failure,
Bunchman et al reported that congenital heart disease, acute tubular necrosis,
sepsis, and bone marrow transplantation were the most common causes.
A retrospective review of 248
patients with a diagnosis of acute renal failure upon discharge or death
revealed acute tubular necrosis and nephrotoxins to be the most common causes
of acute kidney injury.Thus, the etiology of pediatric acute renal failure has
evolved in industrialized countries from primary kidney diseases or prerenal
failure to secondary effects of other systemic illnesses or their treatment.
The pathophysiology of ischemic
acute tubular necrosis is well studied. Ischemia leads to altered tubule cell
metabolism (eg, depletion of adenosine triphosphate [ATP], release of reactive
oxygen species) and cell death, with resultant cell desquamation, cast
formation, intratubular obstruction, backleak of tubular fluid, and oliguria.
Mechanisms of intrinsic acute renal
failure.
In most clinical situations, the
oliguria is reversible and associated with repair and regeneration of tubular
epithelial cells.
Postrenal failure
Postrenal failure is a consequence
of the mechanical or functional obstruction of the flow of urine. This form of
oliguria and renal insufficiency usually responds to the release of the
obstruction.
Principal causes of oliguric
acute kidney injury in neonates
The etiology of oliguria varies with
age, and the common causes in neonates and children are listed separately.
Patients with acute kidney injury secondary to nephrotoxins, interstitial
nephritis, and perinatal asphyxia frequently do not have oliguria.
Prerenal causes include the following:
- Perinatal asphyxia
- Respiratory distress syndrome
- Hemorrhage - Eg, maternal antepartum, twin-twin
transfusion, and intraventricular
- Hemolysis
- Polycythemia
- Sepsis or shock
- Congenital heart disease
- Dehydration
- Drugs - Eg, indomethacin, maternal nonsteroidal
anti-inflammatory drugs (NSAIDs), and maternal ACE inhibitors
Intrinsic renal causes include the
following:
- Acute tubular necrosis
- Exogenous toxins - Eg, aminoglycosides,
amphotericin B, and contrast agents
- Endogenous toxins - Eg, hemoglobin, myoglobin,
and uric acid
- Congenital kidney disease - Eg, agenesis,
polycystic kidney, hypoplasia, and dysplasia
- Vascular - Eg, renal vein thrombosis and renal
artery thrombosis
- Transient renal dysfunction of the newborn
Postrenal causes include the
following:
- Bladder outlet obstruction - Eg, posterior
urethral valves and meatal stenosis
- Neurogenic bladder
- Ureteral obstruction, bilateral
Principal causes of oliguric acute
kidney injury in children
Prerenal causes include the
following:
- Gastrointestinal (GI) losses - Eg, vomiting and
diarrhea
- Blood losses - Eg, hemorrhage
- Renal losses - Eg, diabetes insipidus, diabetes
mellitus, diuretics, and salt-wasting nephropathy
- Cutaneous losses - Eg, burns
- Third space losses - Eg, surgery, trauma,
nephrotic syndrome, and capillary leak
- Shock - Eg, septic, toxic, and anaphylactic
- Impaired autoregulation - Eg, cyclosporine,
tacrolimus, ACE inhibitors, and NSAIDs
- Impaired cardiac output - Eg, congenital and
acquired heart disease
Intrinsic renal
causes include the following:
- Acute tubular necrosis - Eg, prolonged prerenal
failure
- Glomerulonephritis
- Interstitial nephritis, vascular - Eg,
hemolytic-uremic syndrome and vasculitis
- Exogenous toxins - Eg, aminoglycosides,
amphotericin B, cyclosporine, chemotherapy, heavy metals, and contrast
agents
- Endogenous toxins - Eg, hemoglobin, myoglobin,
and uric acid
- Transplant rejection
Postrenal causes
include the following:
- Bladder outlet obstruction - Eg, posterior urethral
valves, blocked catheter, and urethral trauma
- Neurogenic bladder
- Ureteral obstruction, bilateral
Epidemiology
Occurrence in North America
The frequency of oliguria widely
varies depending on the clinical setting. In adults, the incidence is about 1%
at admission, 2-5% during hospitalization, and 4-15% after cardiopulmonary
bypass.
Oliguric acute kidney injury occurs
in approximately 10% of newborn intensive care unit (ICU) patients. The
incidence in children undergoing cardiac surgery is as high as 10-30%. Among
critically ill children admitted to pediatric ICUs (PICUs), the incidence of
acute kidney injury defined by doubling of serum creatinine is present in about
5-6%. This was illustrated by a prospective study from a Canadian PICU that
identified 985 cases of acute kidney injury for an incidence rate of 4.5% of
all PICU admissions.In the largest study reported to date, 3396 admissions to a
single PICU in the United States were retrospectively analyzed.Using serum
creatinine criteria, 6% of children had acute kidney injury on admission and
10% developed acute kidney injury during their PICU stay.
Age-related demographics
Oliguria affects people of all
ages. It is more common in neonatal and older age groups because of comorbid
conditions and is more common in early childhood because of the high incidence
of illnesses that lead to dehydration.
Prognosis
Mortality rates in oliguric acute
kidney injury widely vary according to the underlying cause and associated
medical condition. It ranges from 5% for patients with community-acquired
kidney injury failure to 80% among patients with multiorgan failure in the ICU.
In general, severe acute kidney
injury can have serious short- and long-term consequences. The outcome depends
upon the etiology, age of the child, and comorbidities. In terms of mortality,
severe acute kidney injury requiring renal replacement therapy in children is
still associated with a mortality rate of about 30-50%, and this has not
changed appreciably over the past 3 decades. Infants younger than 1 year have
the highest mortality rate.
In a PICU cohort, patients who
presented with acute kidney injury on admission had a 32% mortality rate, and
those who developed acute kidney injury at any time during the PICU stay had a
30% mortality rate.Additionally, those with any degree of acute kidney injury
at the time of PICU admission had higher PICU mortality than those with normal
kidney function. Moreover, patients who developed any degree of acute kidney
injury during PICU stay had higher ICU mortality than those without acute
kidney injury during PICU stay. Multivariate logistic regression modeling
controlling for age, sex, weight, race, and pediatric index of mortality score
confirmed that acute kidney injury on admission to the PICU was associated with
an increased risk of mortality (adjusted odds ratio, 5.4; 95% CI, 3.5-8.4).
Development of acute kidney injury during the PICU stay was associated with an
even greater risk of mortality (adjusted odds ratio, 8.7; 95% CI, 6.0-12.6) and
a 4-fold increase in length of hospital stay.
In a retrospective analysis of 344
patients from the Prospective Pediatric Continuous Renal Replacement Therapy
(ppCRRT) Registry, the overall mortality rate was 42%.Survival was lowest in
liver disease/transplantation (31%), pulmonary disease/transplantation (45%),
and bone marrow transplantation (45%). Overall survival was better for children
who weighed more than
Thus, it is now clear that patients
die of acute kidney injury and its complications, and not simply with acute
kidney injury.The patient succumbs largely because of involvement of multiple
other systems during the period of severe oliguric renal insufficiency. The
most common causes of death are sepsis and cardiovascular or pulmonary
dysfunction.
Information regarding the long-term
outcome of children after an episode of severe acute kidney injury is scant but
is beginning to accumulate.
In a multicenter pooled analysis of
3476 children with hemolytic uremic syndrome followed for a mean of 4.4
years,the combined average death and end-stage renal disease (ESRD) rate was
12% (95% CI, 10-15%) and the combined average renal sequelae rate (chronic
kidney disease, proteinuria, hypertension) was 25% (95% CI, 20-30%). Thus,
long-term follow-up appears to be warranted after an acute episode of hemolytic
uremic syndrome.
In a retrospective study of 176
children who developed acute kidney injury in a single center, 34% had either
reduced kidney function or were dialysis dependent at hospital discharge.Upon
3-5 years of follow up of the same cohort, the mortality rate was
20%.Approximately 60% developed evidence for chronic kidney disease
(proteinuria, decreased glomerular filtration rate, hypertension) and 9%
developed ESRD.
Collectively, these data strongly
suggest that long-term follow-up is warranted for children who survive an
episode of acute kidney injury.
In contrast to the above, the
prognosis from prerenal causes of acute kidney injury or from acute tubular
necrosis in the absence of significant comorbid conditions is usually quite
good if appropriate therapy is instituted in a timely fashion.
Complications
Infections develop in 30-70% of
patients and affect the respiratory system, urinary tract, and indwelling
catheters. Impaired defenses due to uremia and the inappropriate use of
broad-spectrum antibiotics may contribute to the high rate of infectious
complications.
Cardiovascular complications are a
result of fluid and sodium retention. They include hypertension, congestive
heart failure, and pulmonary edema. Hyperkalemia results in
electrocardiographic abnormalities and arrhythmias.
Other complications include the
following:
- GI - Anorexia, nausea, vomiting, ileus, and
bleeding
- Hematologic - Anemia and platelet dysfunction
- Neurologic - Confusion, asterixis, somnolence,
and seizures
- Other electrolyte/acid-base disorders -
Metabolic acidosis, hyponatremia, hypocalcemia, and hyperphosphatemia
Nursing the child with renal pathology
Techniques
The labels for
urine and stool specimens should contain the following information:
-
the child’s name,
-
the ward unit,
-
the nature of the specimen,
-
the date and hour of collection.
The type of
analysis requested by the doctor routine urine and
stool specimens should be collected in the morning. Since a freshly Passed
specimen facilitates more accurate analysis, specimens should be taken to the
moratory as soon as possible.
In some hospitals,
a disposable cellophane diaper is used to collect urine specimens from infants
and children. The cellophane diaper is applied instead of the regular diaper,
with the point of the diaper between the infant’s legs. The head of the crib
mattress should be elevated in Fowler’s position to facilitate drainage of
urine to the collection portion of the diaper. When the urine specimen has been
passed, the diaper is removed, the point cut with scissors and the urine
transferred to a specimen bottle.
Use of the plastic disposable
urine collector
The type of
plastic disposable urine collector may be affixed to the perineal region to
facilitate the collection of a urine specimen. Peel off the gummed backing (A)
and place the adhesive portion firmly against the perineum of the infant. The
adhesive will adhere to the skin. Place the infant in Fowler's position to aid
the flow of urine by gravity. Check the bag frequently until the desired amount
of urine is obtained. Remove the bag by peeling the adhesive gently from the
skin. Transfer the specimen to a urine bottle, label, and send it to the
laboratory.
Test tube method of collecting
urine
To use a test
tube to collect urine, line the edges of the test tube with adhesive tape.
Insert the penis in the tube and secure the adhesive tape to the pubis. The
infant’s legs should be restrained for safety. Place the infant in Fowler’s
position to aid the flow of urine by gravity. Remove the test tube by peeling
the adhesive from the skin. Transfer the urine to a specimen bottle, label it
and send it to the laboratory. This method should be used only when plastic
disposable urine collectors are not available.
Suprapubic aspiration of urine
Needle
aspiration of urine is used when an adequate clean urine specimen is desired and
other methods have failed. Some pediatricians prefer a suprapublic needle
aspiration to a catheterization procedure. The bladder tap provides unequivocal
information concerning the bacteriology of the urine.
Nursing responsibilities
1. The
procedure should be performed at least one hour after the patient has voided. A
sterile technique is used.
2. The child
lies supine with the legs held in a froglike position.
3. The doctor palpates
the bladder and the nurse may be requested to compress the infant’s urethra.
(In the male infant this is accomplished by pressure on the penis; in the
female, by pressure upward through the rectum). Compression of the urethra
serves to prevent urination during the procedure.
4. The
suprapubic area is cleansed with iodine and alcohol.
6. The
aspirated urine is placed in a sterile tube, labeled, and sent to the
laboratory.
7. No dressing
is required following the procedure.
The nurse
should observe the child for signs of hematuria following the procedure and
report positive findings to the doctor.
Measuring hourly urine output
When the
doctor requests an accurate hourly recording of urine output, and a catheter is
not inserted, the nurse must devise a method to collect all urine passed. A
plastic diaper may be used, with the collecting point of the diaper affixed to
drainage tubing. In male infants, a finger cot may be placed over the penis
(with a hole cut in the end of the finger cot) and the end portion affixed to
drainage tubing. The drainage tubing should be secured to the side of the bed
so that looping of the distal end is avoided and drainage of urine by gravity
is promoted. The distal end of the drainage tubing may empty into a calibrated
drainage bag which is secured to the bedframe.
Renal system
disorders syndromes:
1. Disuria
syndrome
2. Painful
syndrome
3. Urinary
syndrome
4. Edemas
syndrome
5. Hypertension
syndrome
6. Hypotension
syndrome
7. Intoxication
syndrome
8. Nephrotic syndrome
9.
Nephrytyc syndrome
10. Chronic renal failure
11. Acute renal failure
12. Cardiovascular
system dysfunction syndrome
13. Anemic
syndrome
14.
Hemolytic-uremic syndrome
15. Enuresis
(urinary incontinence) syndrome
References
à) Basic
1. Manual of Propaedeutic Pediatrics
/ S.O. Nykytyuk, N.I. Balatska, N.B. Galyash, N.O. Lishchenko, O.Y. Nykytyuk –
Ternopil: TSMU, 2005. – 468 pp.
2. Kapitan T. Propaedeutics of
children’s diseases and nursing of the child : [Textbook for students of higher
medical educational institutions] ; Fourth edition, updated and translated in
English / T. Kapitan –
3. Nelson Textbook of Pediatrics
/edited by Richard E. Behrman, Robert M. Kliegman; senior editor, Waldo E.
Nelson – 19th ed. – W.B.Saunders Company, 2011. – 2680 p.
b) Additional
1.
www.bookfinder.com/author/american-academy-of-pediatrics
2. www.emedicine.medscape.com
3. http://www.nlm.nih.gov/medlineplus/medlineplus.html